1 3 1 2 The Transition Metals Aqa Gcse Chemistry Teaching Resources
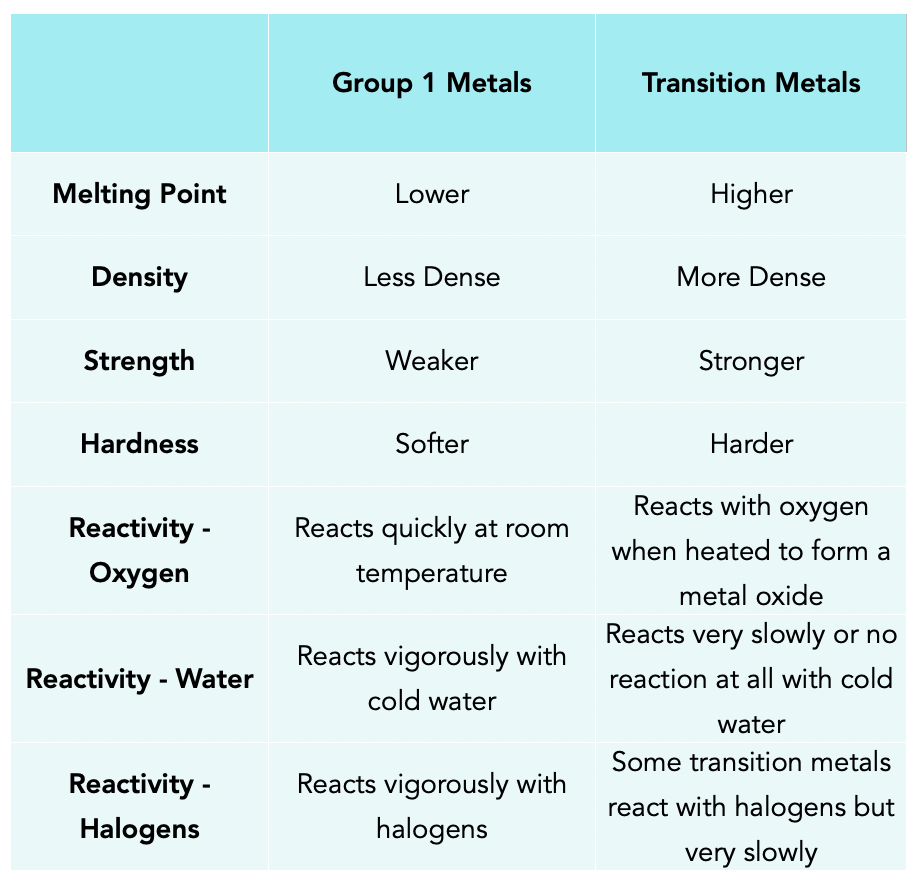
The Transition Metals Gcse Chemistry Study Mind Spec 1.3.1 2 transition metals (recommended teaching time – 1 hour) lesson preparation: demonstration: have some examples of transition metals to show the class. suggested teaching: slide 2: starter questions (differentiated alternative on slide 4) slide 5: periodic table recap questions. slide 7: a recap of the groups of the periodic table. Some properties of transition elements are different from those of the metals in group 1 close group 1 the first vertical column of elements in the periodic table, starting with lithium and ending.
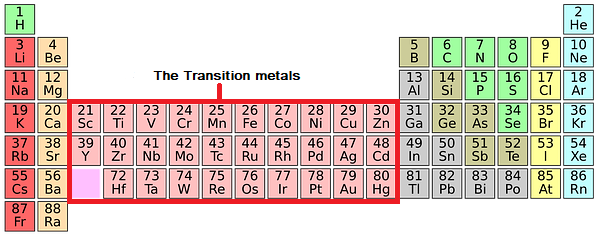
The Transition Metals Chemistry Revision Most of the known metals are transition metals and they have typical properties of metals. they are very lustrous, they are hard, strong and are good conductors of heat and electricity. they are highly dense metals and have very high melting points. transition metals can have more than one oxidation state as they can lose a different number of. Chromium transition elements 1890°c 7.19 g cm 3 manganese transition elements 1240°c 7.20 g cm 3 iron transition elements 1538°c 7.87 g cm 3 cobalt transition elements 1492°c 8.90 g cm 3. The transition metals close transition metal a metal that is located in between group 2 and group 3 (labelled as group 13 on some modern periodic tables) and has brightly coloured compounds. are. Gcse chemistry – the transition metals. transition metals have more than one ion. transition metals form ions with various charges. for example: copper has 2 ions cu or cu 2 , iron has 2 ions fe 2 or fe 3 and cobalt has 2 ions co 2 or co 3 . transition metals can be used as catalysts. transition metals can act as catalysts which means.

Comments are closed.