1 H Nmr 13 C Nmr And Ft Ir Spectra Of Dazd A C And E Tmg Al B D

1 H Nmr 13 C Nmr And Ft Ir Spectra Of Dazd A C And E Tmg Al B D Dazd was synthesized in one step from the 2,2 bis( bromomethyl) propane 1,3 diol in fig. 1a and the structure was con rmed by 1 h 13 c nmr and ft ir spectra shown in fig. 2a, c and e. The basics of 13 c nmr spectroscopy. unlike 1 h nmr signals, the area under a 13 c nmr signal cannot be used to determine the number of carbons to which it corresponds. this is because the signals for some types of carbons are inherently weaker than for other types – peaks corresponding to carbonyl carbons, for example, are much smaller than those for methyl or methylene (ch 2) peaks.

1 H Nmr 13 C Nmr And Ft Ir Spectra Of Dazd A C And E Tmg Al B D 13 c nmr spectral window. one of the greatest advantages of 13 c nmr compared to 1 h nmr is the breadth of the spectrum recall that carbons resonate from 0 220 ppm relative to the tms standard, as opposed to only 0 12 ppm for protons. because of this, 13 c signals rarely overlap, and we can almost always distinguish separate peaks for each. 13.4 chemical shifts in 1 h nmr spectroscopy; 13.5 integration of 1 h nmr absorptions: proton counting; 13.6 spin–spin splitting in 1 h nmr spectra; 13.7 1 h nmr spectroscopy and proton equivalence; 13.8 more complex spin–spin splitting patterns; 13.9 uses of 1 h nmr spectroscopy; 13.10 13 c nmr spectroscopy: signal averaging and ft–nmr. The magnetic moment of a 13 c nucleus is much weaker than that of a proton, meaning that nmr signals from 13 c nuclei are inherently much weaker than proton signals. this, combined with the low natural abundance of 13 c, means that it is much more difficult to observe carbon signals and there is a much lower signal to noise ratio than in 1 h. 1 h n.m.r. spectrum. b has absorptions at 3300 cm–1 and at 1645 cm–1 in its infrared spectrum and does not show e–z isomerism. a b (2) (b) compounds c and d have the molecular formula c 5 h 12 in their 1 h n.m.r. spectra, c has three peaks and d has only one.
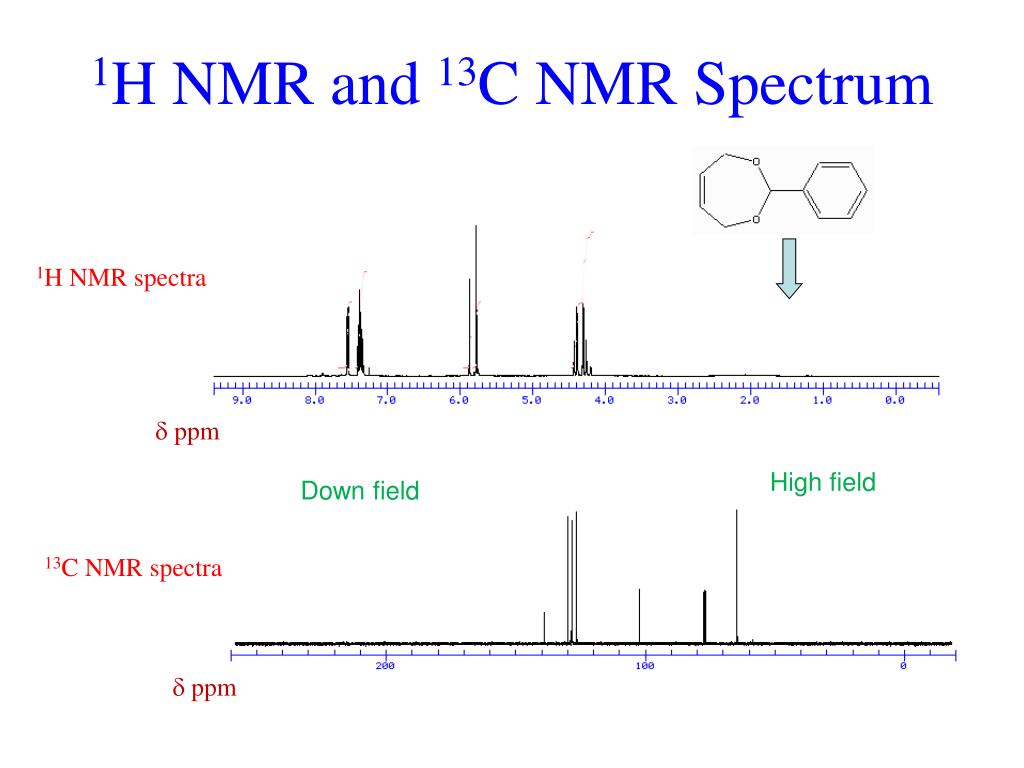
1h Nmr Spectrum The magnetic moment of a 13 c nucleus is much weaker than that of a proton, meaning that nmr signals from 13 c nuclei are inherently much weaker than proton signals. this, combined with the low natural abundance of 13 c, means that it is much more difficult to observe carbon signals and there is a much lower signal to noise ratio than in 1 h. 1 h n.m.r. spectrum. b has absorptions at 3300 cm–1 and at 1645 cm–1 in its infrared spectrum and does not show e–z isomerism. a b (2) (b) compounds c and d have the molecular formula c 5 h 12 in their 1 h n.m.r. spectra, c has three peaks and d has only one. How to read and interpret 1h nmr and 13c. The broad peaks (i.e., 1.56 ppm (peak d) 1.98 ppm (peak c), and 36.13 ppm (peak a) 43.63 ppm (peak b)) observed in nmr spectra were assigned to the protons and carbons of methylene bridge and.
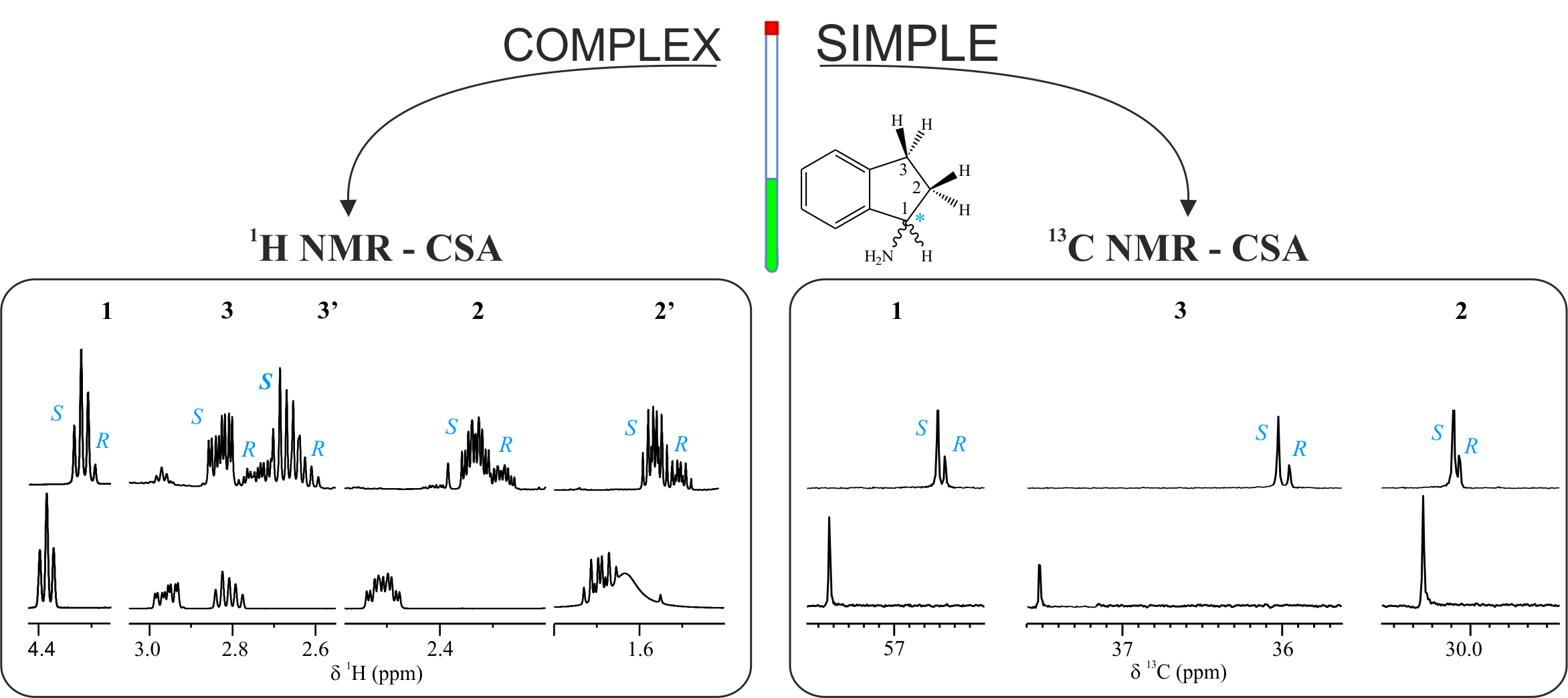
Enantiodifferentiation Through 13c Nmr Spectroscopy And Csas Sermn How to read and interpret 1h nmr and 13c. The broad peaks (i.e., 1.56 ppm (peak d) 1.98 ppm (peak c), and 36.13 ppm (peak a) 43.63 ppm (peak b)) observed in nmr spectra were assigned to the protons and carbons of methylene bridge and.

One Dimensional 1 H Nmr A 13 C Nmr B And Two Dimensional о

Comments are closed.