30a Ranking Acids And Bases By Strength
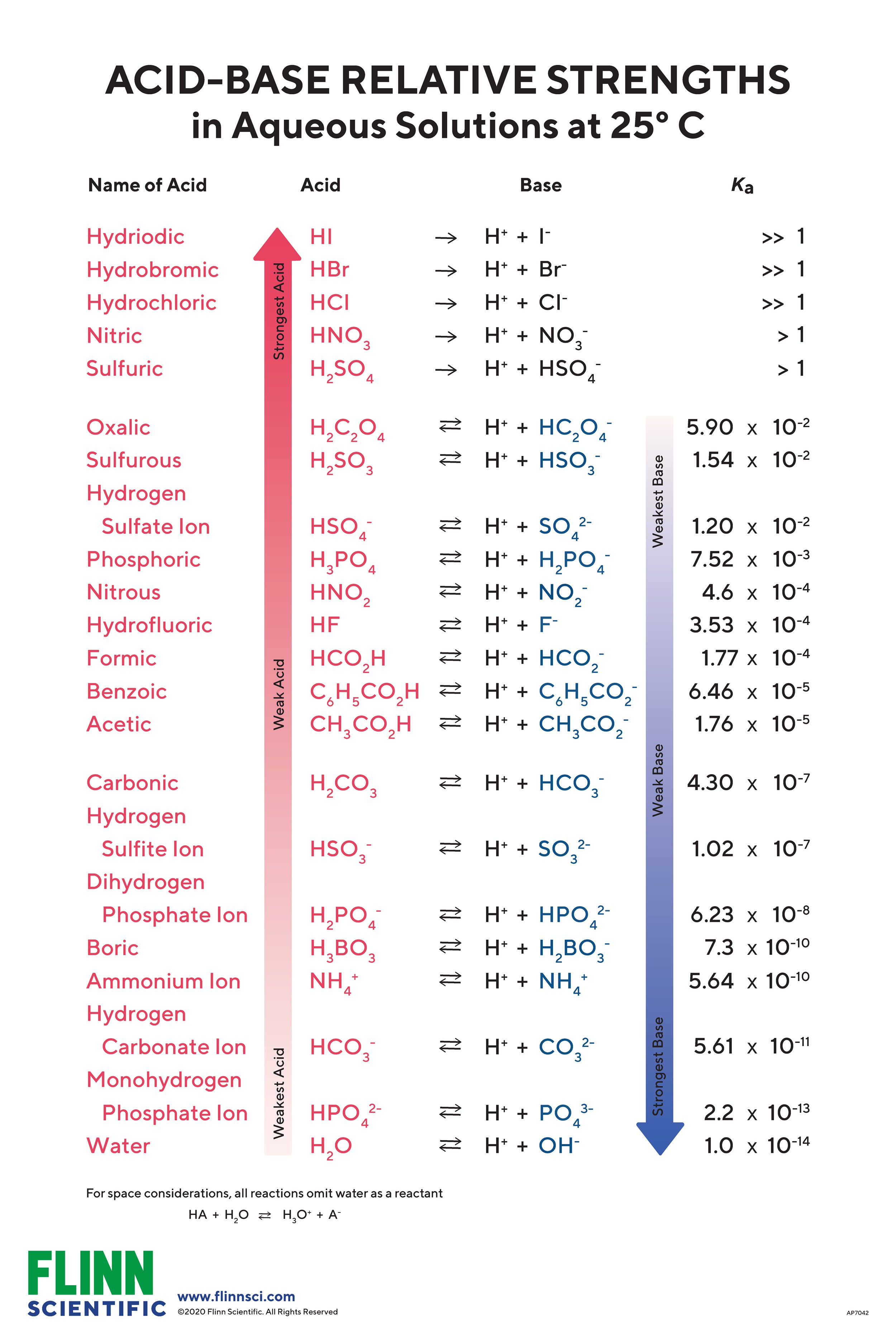
Acid Base Strength Charts For Chemistry Complex ranking of acids and bases. This acid base chart includes the k a value for reference along with the chemical's formula and the acid’s conjugate base. the acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. this chart is ideal for use in the lab or in the classroom.

30a Ranking Acids And Bases By Strength Youtube Factors to assess stability of the conjugate bases when ranking acids’ strength. here are the five factors determining the stability of our conjugate base: resonance: consider acetic acid versus ethanol. if we deprotonate them, we get acetate anion and alkoxide anion respectively. the acetate ion benefits from resonance, spreading the. Acid with values less than one are considered weak. 3. the strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. The first six acids in figure 15.3.3 15.3. 3 are the most common strong acids. these acids are completely dissociated in aqueous solution. the conjugate bases of these acids are weaker bases than water. when one of these acids dissolves in water, their protons are completely transferred to water, the stronger base. B(aq) h 2o(l) ⇌ hb (aq) oh − (aq) water is the acid that reacts with the base, hb is the conjugate acid of the base b, and the hydroxide ion is the conjugate base of water. a strong base yields 100% (or very nearly so) of oh − and hb when it reacts with water; figure 14.3.1 lists several strong bases.
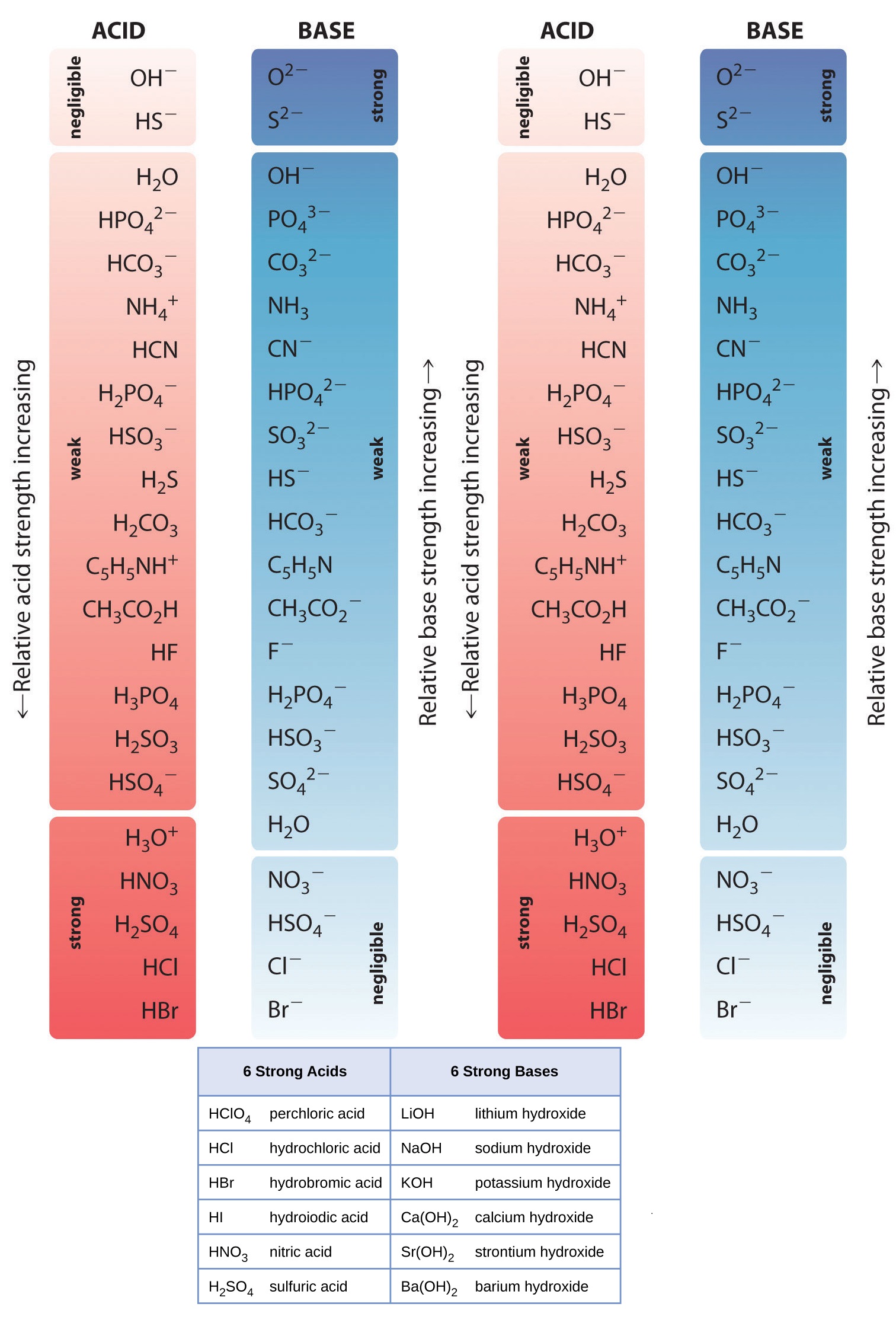
List Of Strong Acids Bases In Order Studypk The first six acids in figure 15.3.3 15.3. 3 are the most common strong acids. these acids are completely dissociated in aqueous solution. the conjugate bases of these acids are weaker bases than water. when one of these acids dissolves in water, their protons are completely transferred to water, the stronger base. B(aq) h 2o(l) ⇌ hb (aq) oh − (aq) water is the acid that reacts with the base, hb is the conjugate acid of the base b, and the hydroxide ion is the conjugate base of water. a strong base yields 100% (or very nearly so) of oh − and hb when it reacts with water; figure 14.3.1 lists several strong bases. Rules for ranking acids and bases (ario) a negative charge raises the energy of electrons (stronger base). a positive charge lowers the energy of electrons (weaker base). the larger the basic atom in a group the more stable the base (weaker base). the more electronegative the basic atom in a period, the more stable the base (weaker base). The relative strengths of strong acids and bases. strong acids, such as hcl, hbr, and hi, all exhibit the same strength in water. the water molecule is such a strong base compared to the conjugate bases cl −, br −, and i − that ionization of these strong acids is essentially complete in aqueous solutions.

Pka Values And Strengths Of Acids And Bases Rules for ranking acids and bases (ario) a negative charge raises the energy of electrons (stronger base). a positive charge lowers the energy of electrons (weaker base). the larger the basic atom in a group the more stable the base (weaker base). the more electronegative the basic atom in a period, the more stable the base (weaker base). The relative strengths of strong acids and bases. strong acids, such as hcl, hbr, and hi, all exhibit the same strength in water. the water molecule is such a strong base compared to the conjugate bases cl −, br −, and i − that ionization of these strong acids is essentially complete in aqueous solutions.
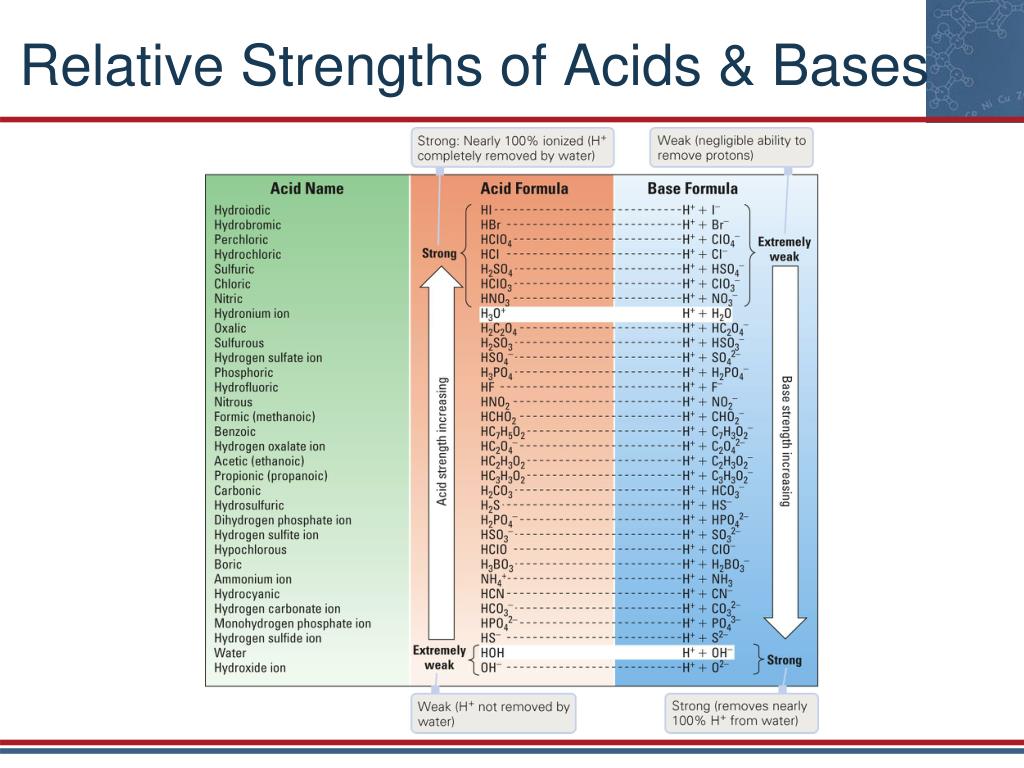
Acid Base Strength Chart

Comments are closed.