4 5 Chemical Symbols And The Atomic Number Chemistry Libretexts

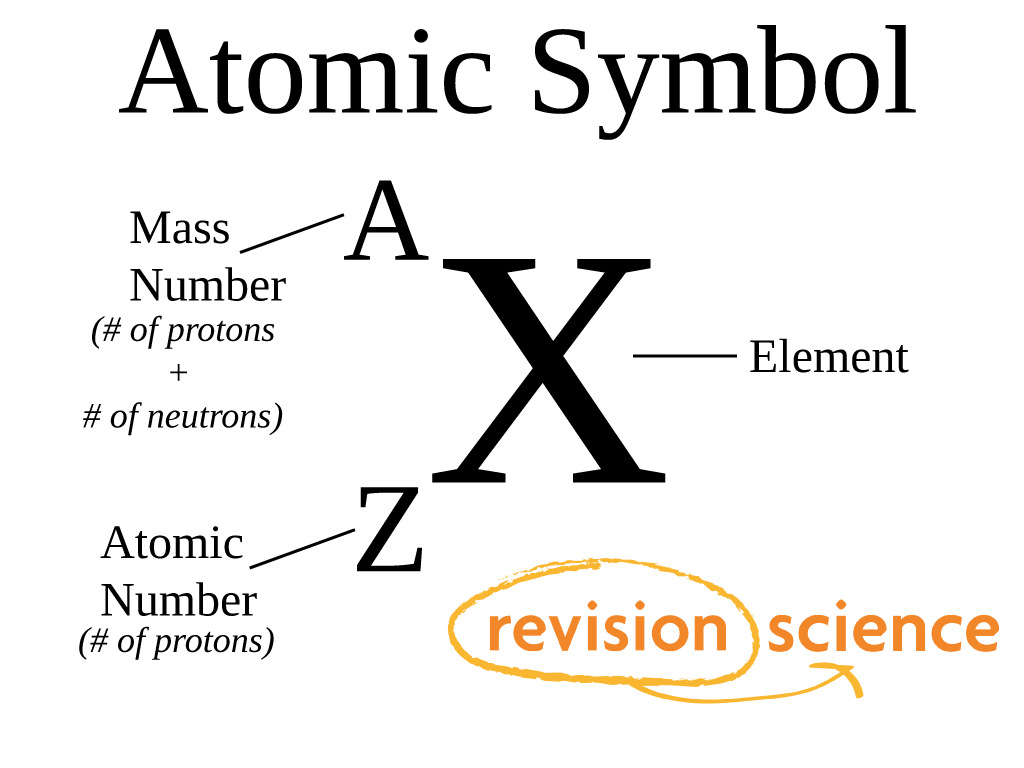
4 5 Chemical Symbols And The Atomic Number Chemistry Li 4.5: chemical symbols and the atomic number is shared under a not declared license and was authored, remixed, and or curated by libretexts. scientists distinguish between different elements by counting the number of protons in the nucleus. since an atom of one element can be distinguished from an atom of another element by the number of …. Figure 2.4.4 2.4. 4: the symbol for an atom indicates the element via its usual two letter symbol, the mass number as a left superscript, the atomic number as a left subscript (sometimes omitted), and the charge as a right superscript. this diagram shows the symbol for helium, “h e.”.

Solved Chemical Symbols Complete The Following Table By Chegg The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. an amu is defined as exactly of the mass of a carbon 12 atom and is equal to 1.6605 × 10 −24 g. protons are relatively heavy particles with a charge of 1 and a mass of 1.0073 amu. In addition to providing the atomic number for each element, the periodic table also displays the element’s atomic mass. looking at carbon, for example, its symbol (c) and name appear, as well as its atomic number of six (in the upper left hand corner) and its atomic mass of 12.11. figure 1. An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. the positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. most of an atom's mass is in its nucleus; the mass of an electron is only 1 1836 the mass of the lightest. This name describes the atomic number of the element, followed by the ium suffix. for example, element 120 has the temporary name of unbinilium. the temporary names are cumbersome, so it’s perfectly acceptable to refer to an unverified element by its atomic number (e.g., element 122, element 145).
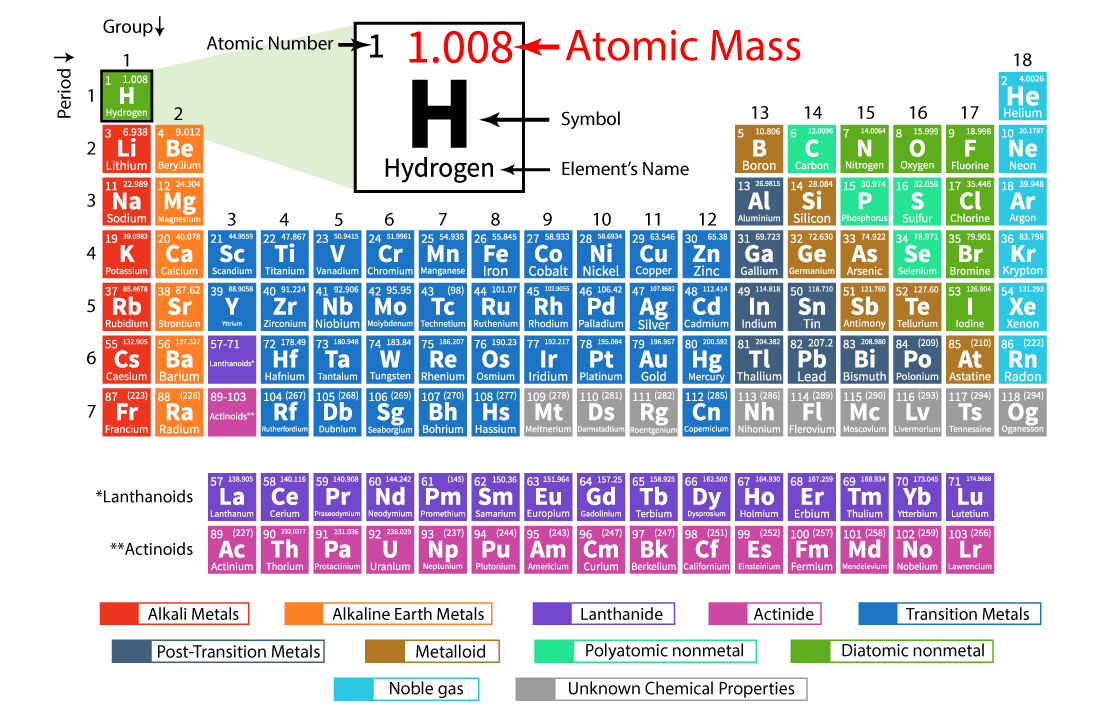
First Elements Of The Periodic Table With Atomic Number And Mass My An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. the positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. most of an atom's mass is in its nucleus; the mass of an electron is only 1 1836 the mass of the lightest. This name describes the atomic number of the element, followed by the ium suffix. for example, element 120 has the temporary name of unbinilium. the temporary names are cumbersome, so it’s perfectly acceptable to refer to an unverified element by its atomic number (e.g., element 122, element 145). 2.4: nuclei of atoms elements can be identified by their atomic number and mass number. isotopes are atoms of the same element that have different masses. 2.5: atomic masses atoms have a mass that is based largely on the number of protons and neutrons in their nucleus. 2.6: arrangements of electrons. In addition to providing the atomic number for each element, the periodic table also displays the element’s atomic mass. looking at carbon, for example, its symbol (c) and name appear, as well as its atomic number of six (in the upper left hand corner) and its atomic mass of 12.11.
Question Fab79 Socratic 2.4: nuclei of atoms elements can be identified by their atomic number and mass number. isotopes are atoms of the same element that have different masses. 2.5: atomic masses atoms have a mass that is based largely on the number of protons and neutrons in their nucleus. 2.6: arrangements of electrons. In addition to providing the atomic number for each element, the periodic table also displays the element’s atomic mass. looking at carbon, for example, its symbol (c) and name appear, as well as its atomic number of six (in the upper left hand corner) and its atomic mass of 12.11.

Comments are closed.