5 4 Linear Regression And Calibration Curves Chemistry Libretexts
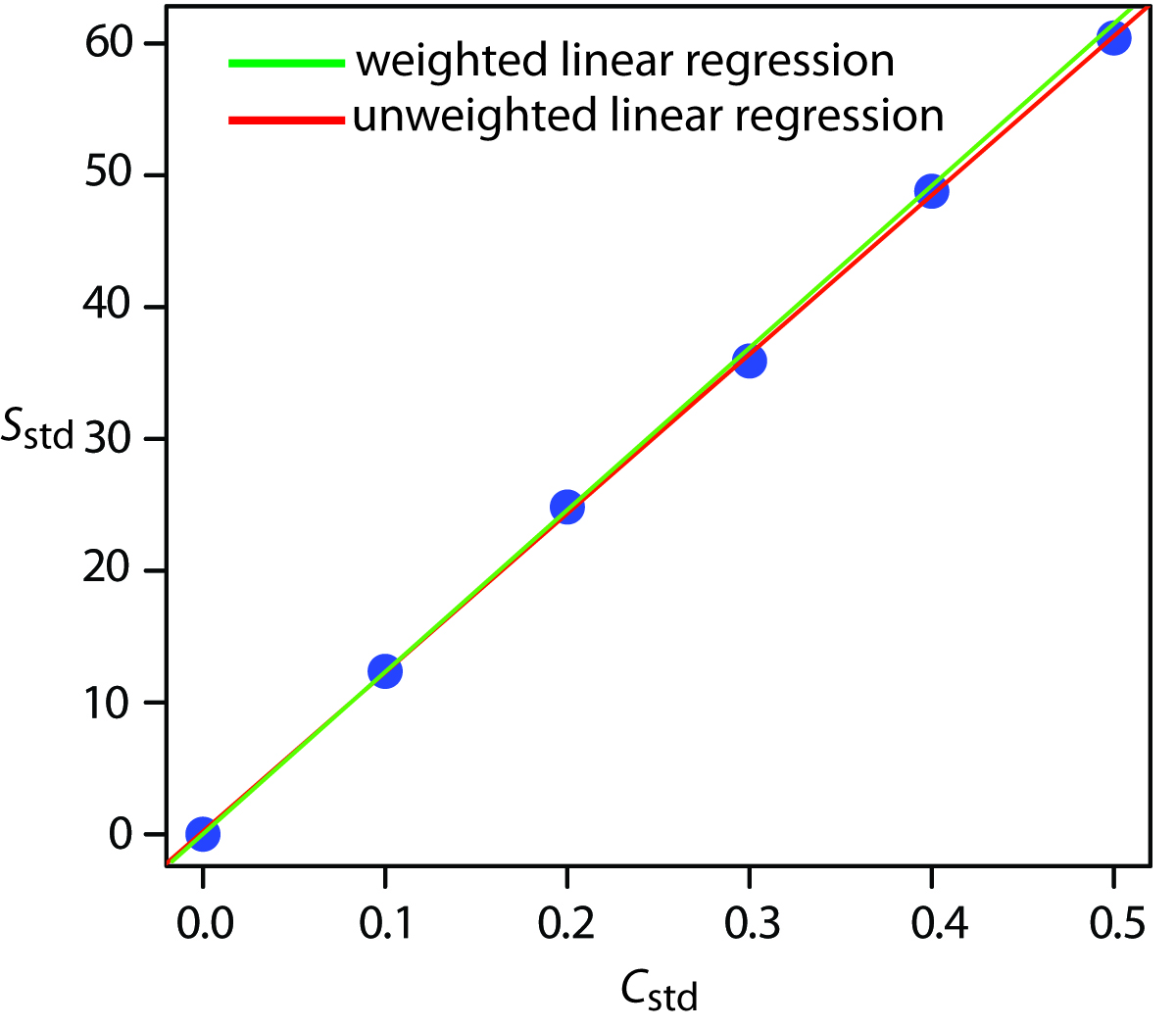
5 4 Linear Regression And Calibration Curves Chemistry Figure 5.4.1 shows the data in table 5.4.1 plotted as a normal calibration curve. although the data certainly appear to fall along a straight line, the actual calibration curve is not intuitively obvious. the process of determining the best equation for the calibration curve is called linear regression. The libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. we also acknowledge previous national science foundation support.
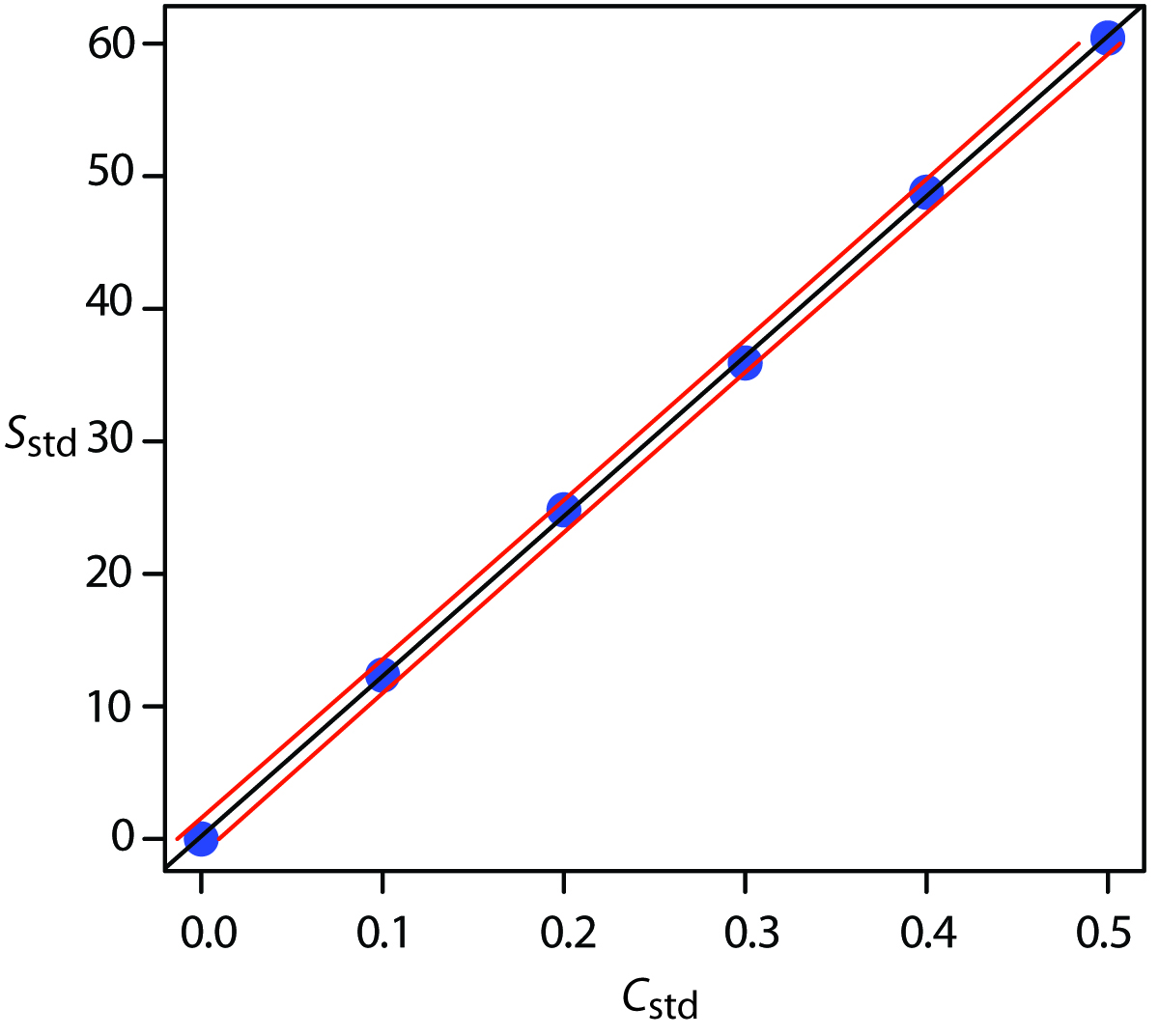
5 4 Linear Regression And Calibration Curves Chemistry Legal. accessibility statement for more information contact us at [email protected]. in a single point external standardization we determine the value of \ (k a\) by measuring the signal for a single standard containing a known concentration of analyte. using this value of \ (k a\) and …. 5.4: linear regression and calibration curves 5.5: blank corrections 5.6: using excel and r for a regression analysis 5.7: standardizing analytical methods (exercises) 5.8: standardizing analytical methods (summary) 6: equilibrium chemistry 6.1: reversible reactions and chemical equilibria 6.2: thermodynamics and equilibrium chemistry. Calibration and linear regression analysis: a self guided tutorial (part 2) chm314 instrumental analysis, dept. of chemistry, univ. of toronto d. stone, j. ellis 2 1.1 the correlation coefficient in part 1 of the tutorial, we saw how to use the trendline feature in excel to fit a straight line through. Calibration curve is a regression model used to predict the unknown concentrations of analytes of interest based on the response of the instrument to the known standards. some statistical analyses are required to choose the best model fitting to the experimental data and also evaluate the linearity and homoscedasticity of the calibration curve. using an internal standard corrects for the loss.
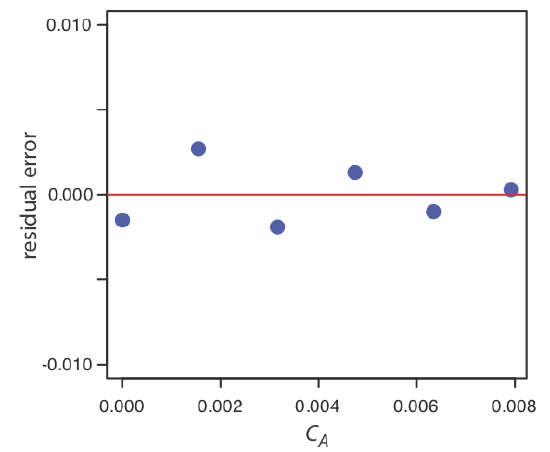
5 4 Linear Regression And Calibration Curves Chemistry Calibration and linear regression analysis: a self guided tutorial (part 2) chm314 instrumental analysis, dept. of chemistry, univ. of toronto d. stone, j. ellis 2 1.1 the correlation coefficient in part 1 of the tutorial, we saw how to use the trendline feature in excel to fit a straight line through. Calibration curve is a regression model used to predict the unknown concentrations of analytes of interest based on the response of the instrument to the known standards. some statistical analyses are required to choose the best model fitting to the experimental data and also evaluate the linearity and homoscedasticity of the calibration curve. using an internal standard corrects for the loss. Every calibration curve is defined by a set of parameters: in the case of linear calibration curves, they are usually: the slope of the line (its angle in relation to the horizontal axis); and; the intercept. to find out these parameters, you need to measure the signal obtained from a set of samples with known concentrations. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. the stock solution of naoh is 10%. draw diagram as part of your description. using the standard curve below, calculate the concentration of an unknown solution if its absorbance is 0.55. figure 3.
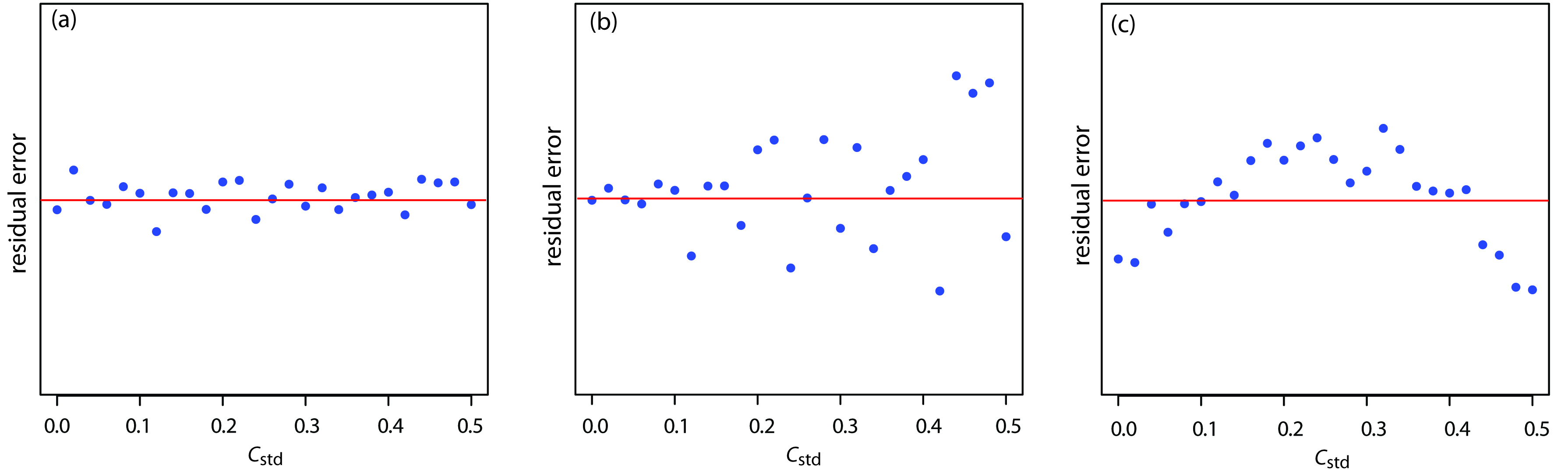
5 4 Linear Regression And Calibration Curves Chemistry Every calibration curve is defined by a set of parameters: in the case of linear calibration curves, they are usually: the slope of the line (its angle in relation to the horizontal axis); and; the intercept. to find out these parameters, you need to measure the signal obtained from a set of samples with known concentrations. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. the stock solution of naoh is 10%. draw diagram as part of your description. using the standard curve below, calculate the concentration of an unknown solution if its absorbance is 0.55. figure 3.

Comments are closed.