An Overview Of New Drug Discovery And Development Pharmatutor
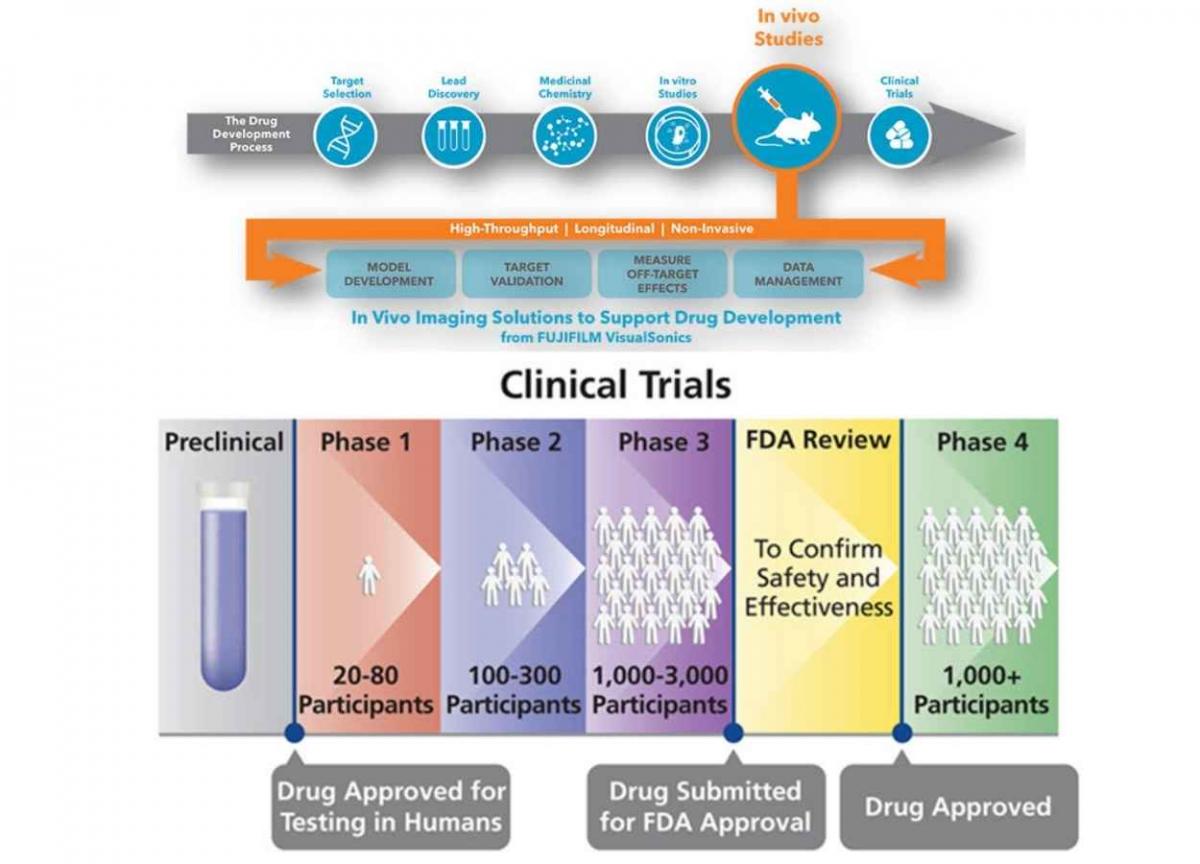
An Overview Of New Drug Discovery And Development Pharmatutor The drug discovery and development of new medicines is a long completed process. research based pharmaceutical companies are committed to advancing science and bringing new medicines to patients. increased support from government and organization may helps in development safer and cost effective medicines.s. reference: 1. Computer aided drug design (cadd) provides a variety of tools and techniques that assist in the various stages of drug design, thereby reducing the cost of drug research and development time. drug discovery and the development of a new drug is a long, complex, costly and highly risky process that has no equal in the commercial world.
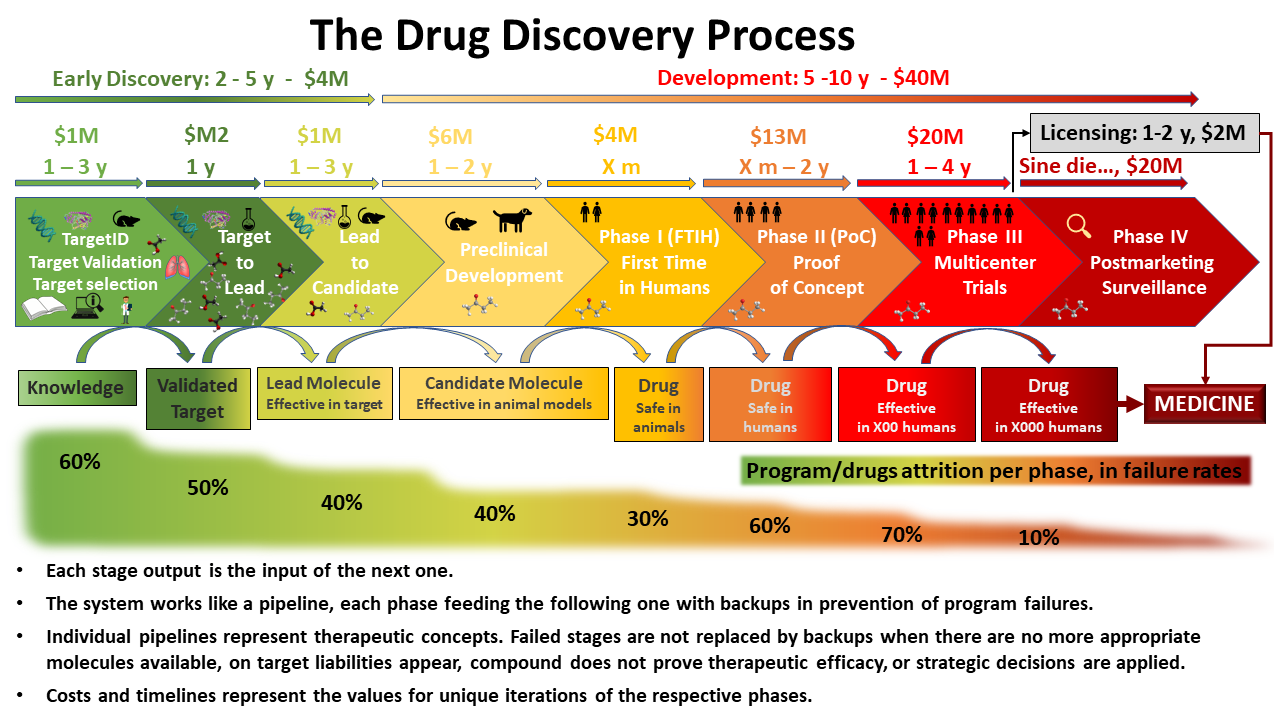
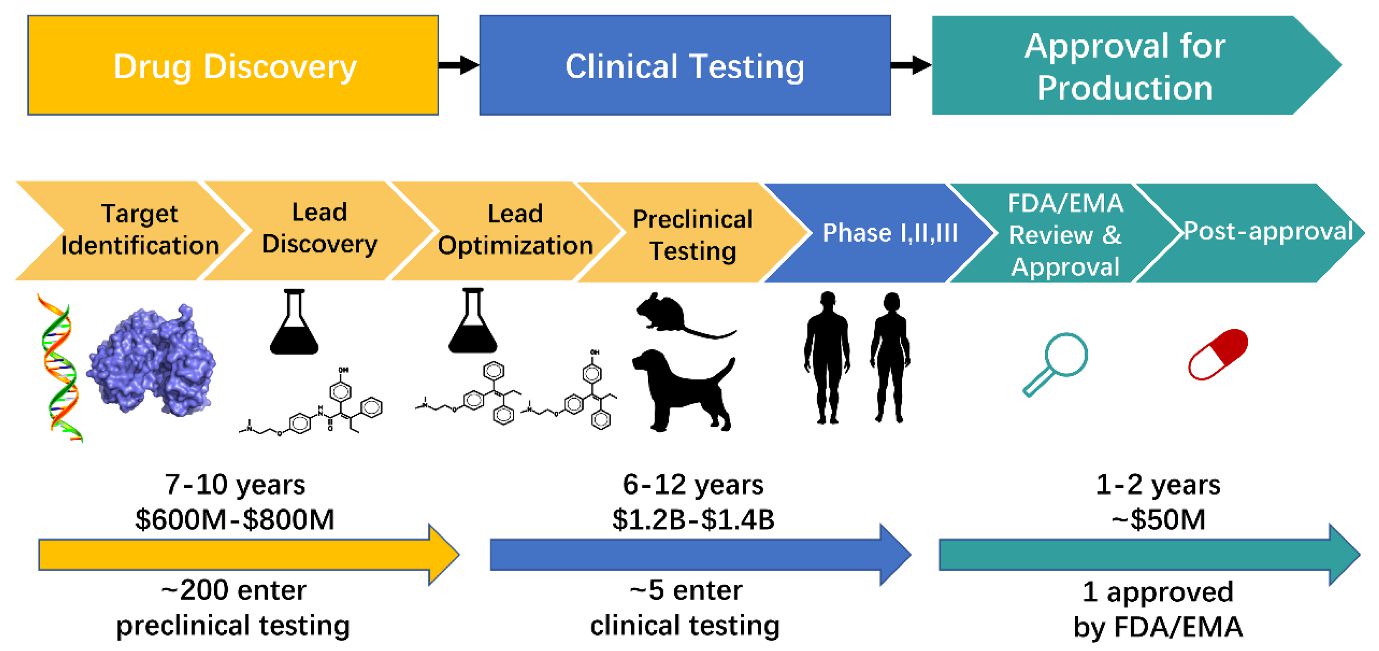
Drug Discovery Target Identification Abstract. a new medicine will take an average of 10 15 years and more than us$2 billion before it can reach the pharmacy shelf. traditionally, drug discovery relied on natural products as the main source of new drug entities, but was later shifted toward high throughput synthesis and combinatorial chemistry based development. Reference id: pharmatutor art 1316 introduction as the pharmaceutical industries throughout the world are moving ahead towards becoming more and more competitive, regulatory agencies are being established in various countries across the globe.regulatory authority and organizations are responsible in effective drug regulation required to ensure the safety, efficacy and quality of drugs, as well. T he process of creating a new drug product can be broadly divided into three main phases: drug discovery – entailing the conceptualisation of the therapeutic into a molecule with known pharmacologic effects; drug development – covering the steps taken to convert the molecule above into an approved and registered drug product. Abstract. a new medicine will take an average of 10–15 years and more than us$2 billion before it can reach the pharmacy shelf. traditionally, drug discovery relied on natural products as the main source of new drug entities, but was later shifted toward high throughput synthesis and combinatorial chemistry based development.

Drug Development Process Overview T he process of creating a new drug product can be broadly divided into three main phases: drug discovery – entailing the conceptualisation of the therapeutic into a molecule with known pharmacologic effects; drug development – covering the steps taken to convert the molecule above into an approved and registered drug product. Abstract. a new medicine will take an average of 10–15 years and more than us$2 billion before it can reach the pharmacy shelf. traditionally, drug discovery relied on natural products as the main source of new drug entities, but was later shifted toward high throughput synthesis and combinatorial chemistry based development. Overview of drug development process. cmc, chemistry, manufacturing and controls; mtd, maximum tolerated dose; ind, investigated new drug application; nda, new drug application. the discovery and development of innovative drugs is time and cost intensive and currently approximately twelve years and an average of $1.8 billion is required to. At this stage, the project focus shifts from drug discovery to drug development to enable human clinical trials. if the therapeutic agent is successful in all three phases of the clinical trials, it goes through regulatory registration and the drug can be marketed ( hefti, 2008; hughes et al., 2011; mohs and greig, 2017 ).

关于我们 Overview of drug development process. cmc, chemistry, manufacturing and controls; mtd, maximum tolerated dose; ind, investigated new drug application; nda, new drug application. the discovery and development of innovative drugs is time and cost intensive and currently approximately twelve years and an average of $1.8 billion is required to. At this stage, the project focus shifts from drug discovery to drug development to enable human clinical trials. if the therapeutic agent is successful in all three phases of the clinical trials, it goes through regulatory registration and the drug can be marketed ( hefti, 2008; hughes et al., 2011; mohs and greig, 2017 ).
How Cadd Computational Aided Drug Design Help In Drug Discovery

Comments are closed.