Ap04 15 Silver Sulfide Molecular And Net Ionic Reactions

Ap04 15 Silver Sulfide Molecular And Net Ionic Reactions Youtube Metathesis reaction of sodium sulfide and silver nitrate. ap chemistry. Enter an equation of an ionic chemical equation and press the balance button. the balanced equation will be calculated along with the solubility states, complete ionic equation, net ionic equation, spectator ions and precipitates. use uppercase for the first character in the element and lowercase for the second character.

Ap04 15 Strong Acid Strong Base Net Ionic Reactions Youtube Do you want to learn how to write molecular, complete ionic, and net ionic equations for chemical reactions? khan academy offers a free, interactive video lesson that explains the steps and rules for each type of equation. you will also see examples and practice problems to test your understanding. join khan academy and discover the world of chemistry with fun and engaging courses. To show the overall chemical transformation, we omit the spectator ions, and the resulting equation is called the net ionic equation: 3ba 2 (aq) 2po 4 3 (aq) → ba 3 (po4) 2 (s) check also. solutions; strong and weak electrolytes; dissociation of ionic compounds; molarity dilution; ion concentration; precipitation reactions; definitions of. Here are the word equations above, repeated using formulas: hcl (aq) naoh (aq) > nacl (aq) h 2 o (ℓ) only full formulas (never words or ions) are involved in a complete molecular equation. ions (never words) will be used for the complete ionic equation and the net ionic equation, which will follow just below. In the above reaction, the sodium ion and the nitrate ion are both spectator ions. the equation can now be written without the spectator ions: ag (aq) cl− (aq) → agcl(s) ag ( a q) cl − ( a q) → agcl ( s) the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved.
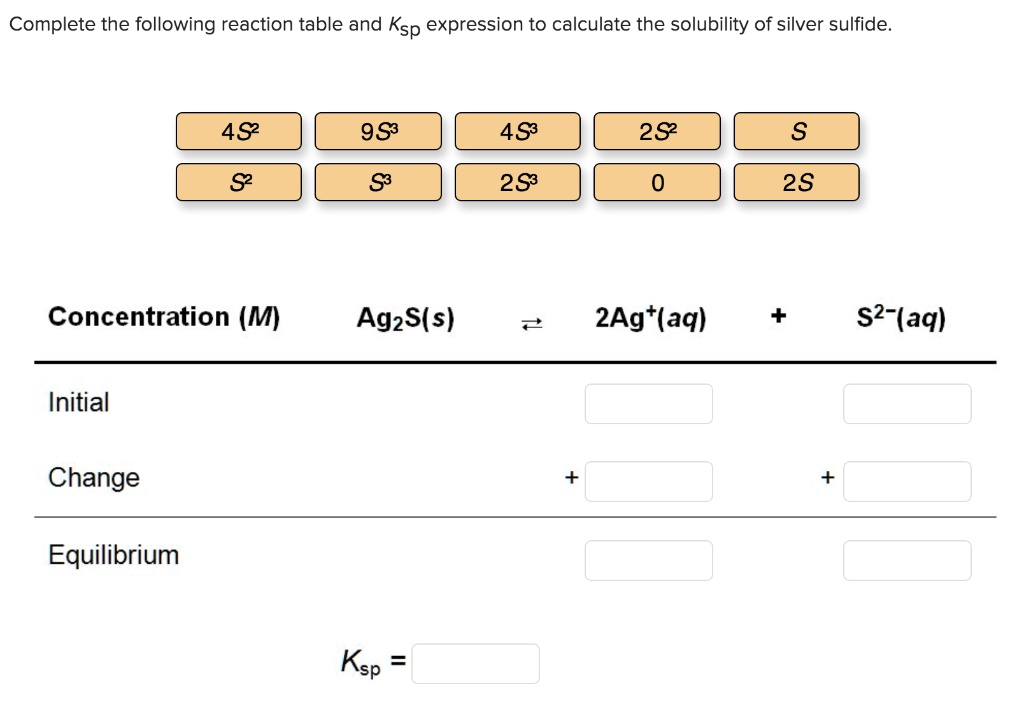
Solved Complete The Following Reaction Table And Ksp Expression To Here are the word equations above, repeated using formulas: hcl (aq) naoh (aq) > nacl (aq) h 2 o (ℓ) only full formulas (never words or ions) are involved in a complete molecular equation. ions (never words) will be used for the complete ionic equation and the net ionic equation, which will follow just below. In the above reaction, the sodium ion and the nitrate ion are both spectator ions. the equation can now be written without the spectator ions: ag (aq) cl− (aq) → agcl(s) ag ( a q) cl − ( a q) → agcl ( s) the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved. Exercise 12.1.1. write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reaction of aqueous silver fluoride with aqueous sodium phosphate to give solid silver phosphate and a solution of sodium fluoride. answer: overall chemical equation: 3agf(aq) na3po4(aq) → ag3po4(s) 3naf(aq) (12.1.11). Exercise 8.11.1 8.11. 1. write the chemical equation that represents the dissociation of (nh 4) 2 s. answer. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. a complete ionic equation is a chemical equation in.

How To Write A Molecular Exercise 12.1.1. write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reaction of aqueous silver fluoride with aqueous sodium phosphate to give solid silver phosphate and a solution of sodium fluoride. answer: overall chemical equation: 3agf(aq) na3po4(aq) → ag3po4(s) 3naf(aq) (12.1.11). Exercise 8.11.1 8.11. 1. write the chemical equation that represents the dissociation of (nh 4) 2 s. answer. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. a complete ionic equation is a chemical equation in.
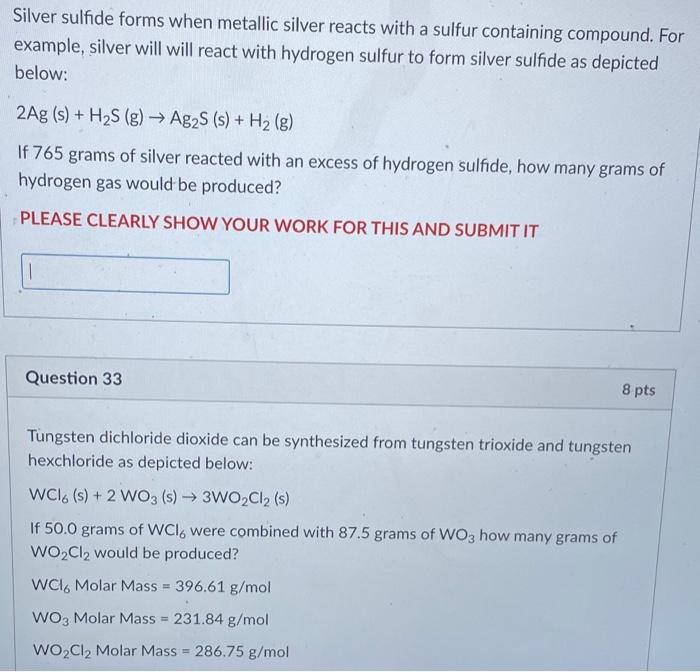
Solved Silver Sulfide Forms When Metallic Silver Reacts With Chegg

Comments are closed.