Basic Chemistry For Biology Part 4 Covalent Bonding And Structural Formulas
Basic Chemistry For Biology Part 4 Covalent Bonding And This video series, basic chemistry for biology students, teaches the basic chemistry that you’ll need to know in your biology course, whether that’s introduc. These bonds much more common than ionic bonds in the molecules of living organisms. covalent bonds are commonly found in carbon based organic molecules, such as our dna and proteins. covalent bonds are also found in inorganic molecules like h 2 o, co 2, and o 2. one, two, or three pairs of electrons may be shared, making single, double, and.

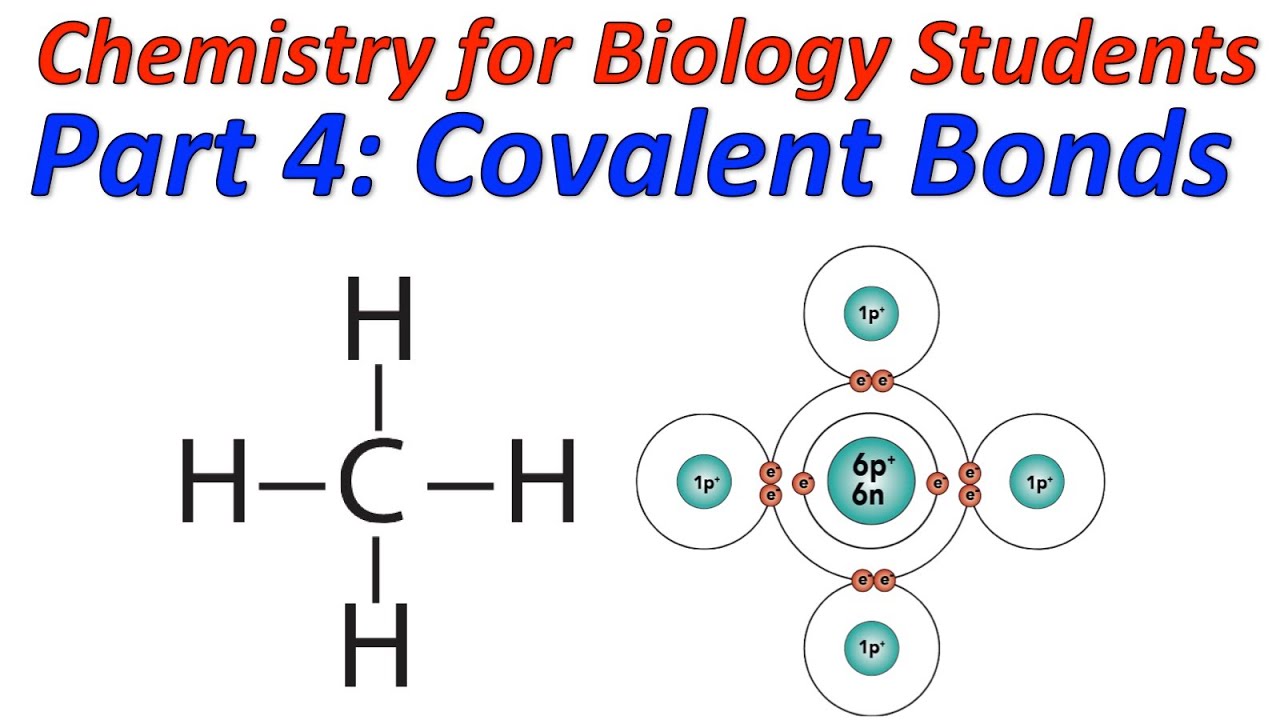
Covalent Bond Types This type of bonding would be a covalent bond. two combinations of atoms can produce this type of bonding: nonmetal nonmetal or metalloid nonmetal. in this class, we will not discuss the option of metallic bonding which is a form of covalent bonding. figure 4.8.1 4.8. 1: sharing is caring, especially for atoms that participate in covalent. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2−. the formula of the carbonate ion is co 32−. the atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. several examples are found in table 3.3.1. This bond, formed by sharing a pair of electrons, is called a covalent bond. 1.2. covalent bonding in methane, ch4. let’s look at a slightly more complex molecule. the molecule is methane (a common fuel), and its formula is ch4. you know that carbon has 6 protons, 6 neutrons, and 6 electrons. This is because it has only one shell and this shell can only hold 2 electrons. 8. bonding electrons: 4; nonbonding electrons: 4. 9. bonding electrons: 8; nonbonding electrons: 24. 10. bonding electrons: 6; nonbonding electrons: 20. 11. hydrogen atoms form only one covalent bond because they have only one valence electron to pair.

Covalent Bond Definition Types And Examples This bond, formed by sharing a pair of electrons, is called a covalent bond. 1.2. covalent bonding in methane, ch4. let’s look at a slightly more complex molecule. the molecule is methane (a common fuel), and its formula is ch4. you know that carbon has 6 protons, 6 neutrons, and 6 electrons. This is because it has only one shell and this shell can only hold 2 electrons. 8. bonding electrons: 4; nonbonding electrons: 4. 9. bonding electrons: 8; nonbonding electrons: 24. 10. bonding electrons: 6; nonbonding electrons: 20. 11. hydrogen atoms form only one covalent bond because they have only one valence electron to pair. Ch4.1 covalent bond. in ionic compounds, electrons are transferred between atoms of different elements to form ions. but this is not the only way that compounds can be formed. atoms can also make chemical bonds by sharing electrons between each other. such bonds are called . Types of covalent bonds in the ethane lewis formula shown above all bonds are represented as single lines called single bonds. each single bond is made up of two electrons, called bonding electrons. it is also possible for two atoms bonded together to share 4 electrons. this bonding pattern is represented by two lines, each representing two.

Comments are closed.