Bms 986165 Dose 1 For Ulcerative Colitis Clinical Trial 2023 Power

Bms 986165 Dose 1 For Ulcerative Colitis Clinical Trial 2023 Power Methotrexate (mtx), a substrate of organic anion transporter 1 and 3, is an immunosuppressive agent recommended for the treatment of steroid dependent ulcerative colitis and active relapsing crohn’s disease. 1,2 bms 986165, an oral selective tyk2 inhibitor, has demonstrated efficacy and acceptable safety in patients with moderate to severe plaque psoriasis, 3 and is under investigation in. Page: 1 protocol number: im011024 date; version: 14 apr 2021, protocol amendment 03 clinical protocol im011024 a phase 2 randomized, double blind, placebo controlled study of thesafety and efficacy of bms 986165 in subjects with moderate to severe ulcerative colitis short title: safety and efficacy of bms 986165 in subjects with moderate to severe.
:%0A%0ABMS-986165 Dose 2 oral administration for Lupus.png?md=1)
Bms 986165 Dose 2 Oral Administration For Lupus Clinical Trial 2023 February 9, 2024 updated by: bristol myers squibb. a phase 2 randomized, double blind, placebo controlled study of the safety and efficacy of bms 986165 in subjects with moderate to severe ulcerative colitis. the purpose of this study is to assess the safety and efficacy of oral deucravacitinib in participants with moderate to severe ulcerative. Background tyrosine kinase 2 (tyk2) is a member of the jak family that phosphorylates stat proteins downstream of the il 12, il 23 and the type i interferon receptor. tyk2 genetic variants have been linked to multiple autoimmune diseases,1 with a deactivating coding variant conferring significant protection against multiple immune mediated disorders including systemic lupus erythematosus (sle. Study nct04613518 submitted date: november 2, 2023 (v28) study identification. unique protocol id: im011 127. brief title: a study of the safety, efficacy, and biomarker response of bms 986165 in participants with moderate to severe ulcerative colitis. official title:. The purpose of this study is to evaluate the long term safety and efficacy of bms 986165 in participants who have previously been enrolled in a bms 986165 phase 2 study for moderate to severe crohn's disease or moderate to severe ulcerative colitis. detailed description:.

Sat0226 A First In Human Study Of Bms 986165 A Selective Potent Study nct04613518 submitted date: november 2, 2023 (v28) study identification. unique protocol id: im011 127. brief title: a study of the safety, efficacy, and biomarker response of bms 986165 in participants with moderate to severe ulcerative colitis. official title:. The purpose of this study is to evaluate the long term safety and efficacy of bms 986165 in participants who have previously been enrolled in a bms 986165 phase 2 study for moderate to severe crohn's disease or moderate to severe ulcerative colitis. detailed description:. Bms 986165 is still being studied, so not all of its potential side effects are known. the most common side effects seen in a recent phase 2 clinical trial of bms 986165 in psoriasis, a skin disease, were irritation of the nose and throat (nasopharyngitis), headache, diarrhea, nausea, and upper respiratory tract infection. In a model of inflammatory bowel disease, colitis was induced in scid mice by an agonistic anti cd40 ab, and effect of bms 986165 (administered po qd) was evaluated against both the body weight loss and histologically evident colitis. results: bms 986165 potently binds to the tyk2 pseudokinase domain (ki = 0.02 nm), and is highly selective.
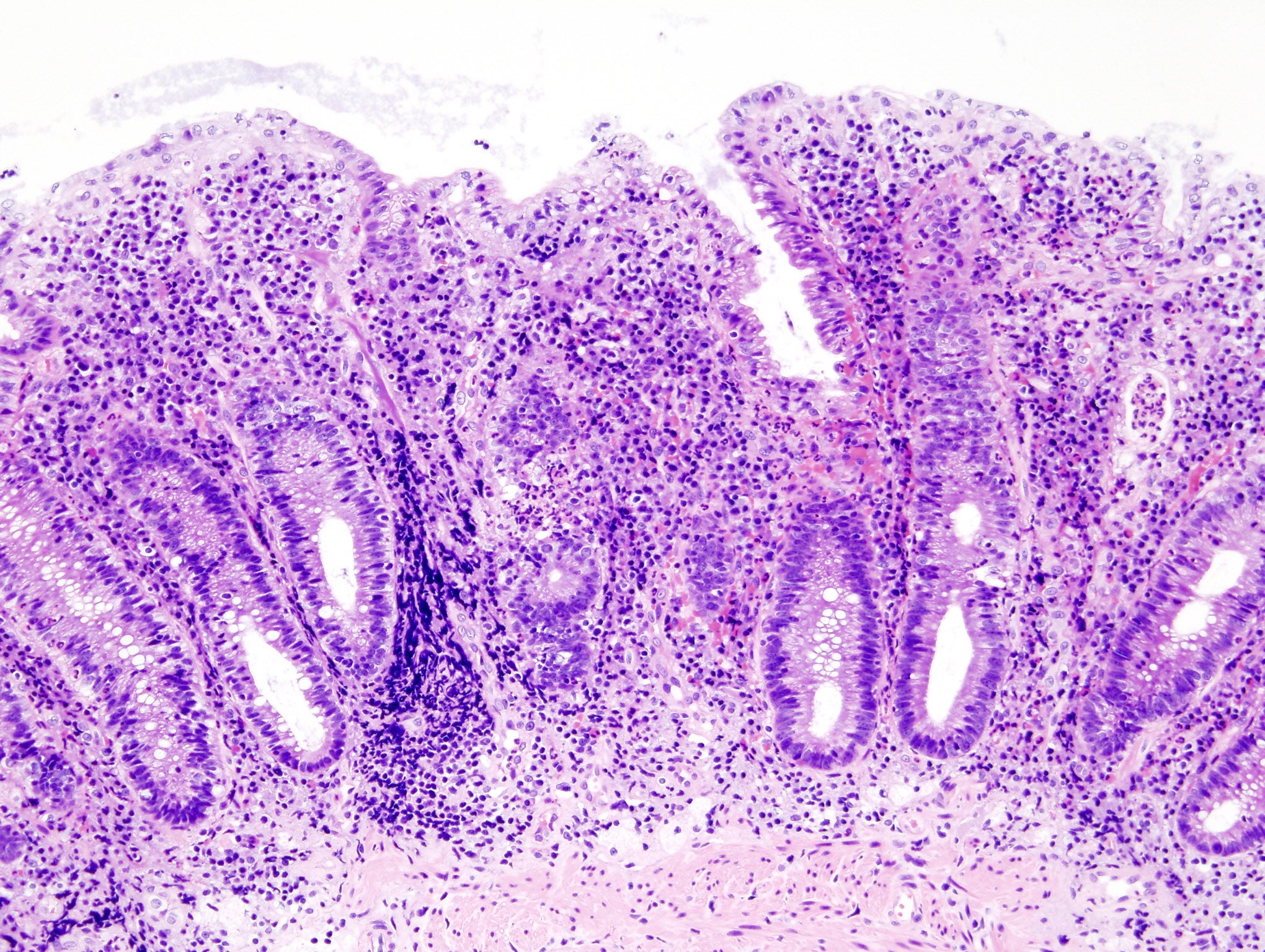
юааbmsюабтащ Deucravacitinib Fails To Meet Efficacy Goals In юааulcerativeюаб юааcolitisюаб Bms 986165 is still being studied, so not all of its potential side effects are known. the most common side effects seen in a recent phase 2 clinical trial of bms 986165 in psoriasis, a skin disease, were irritation of the nose and throat (nasopharyngitis), headache, diarrhea, nausea, and upper respiratory tract infection. In a model of inflammatory bowel disease, colitis was induced in scid mice by an agonistic anti cd40 ab, and effect of bms 986165 (administered po qd) was evaluated against both the body weight loss and histologically evident colitis. results: bms 986165 potently binds to the tyk2 pseudokinase domain (ki = 0.02 nm), and is highly selective.
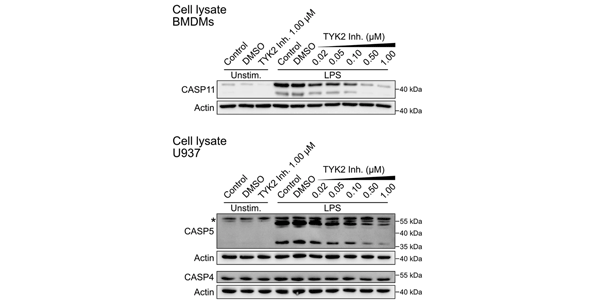
Deucravacitinib Bms 986165 Tyk2 Inhibitor Medchemexpress

Comments are closed.