Calibration Curves вђ Part 1
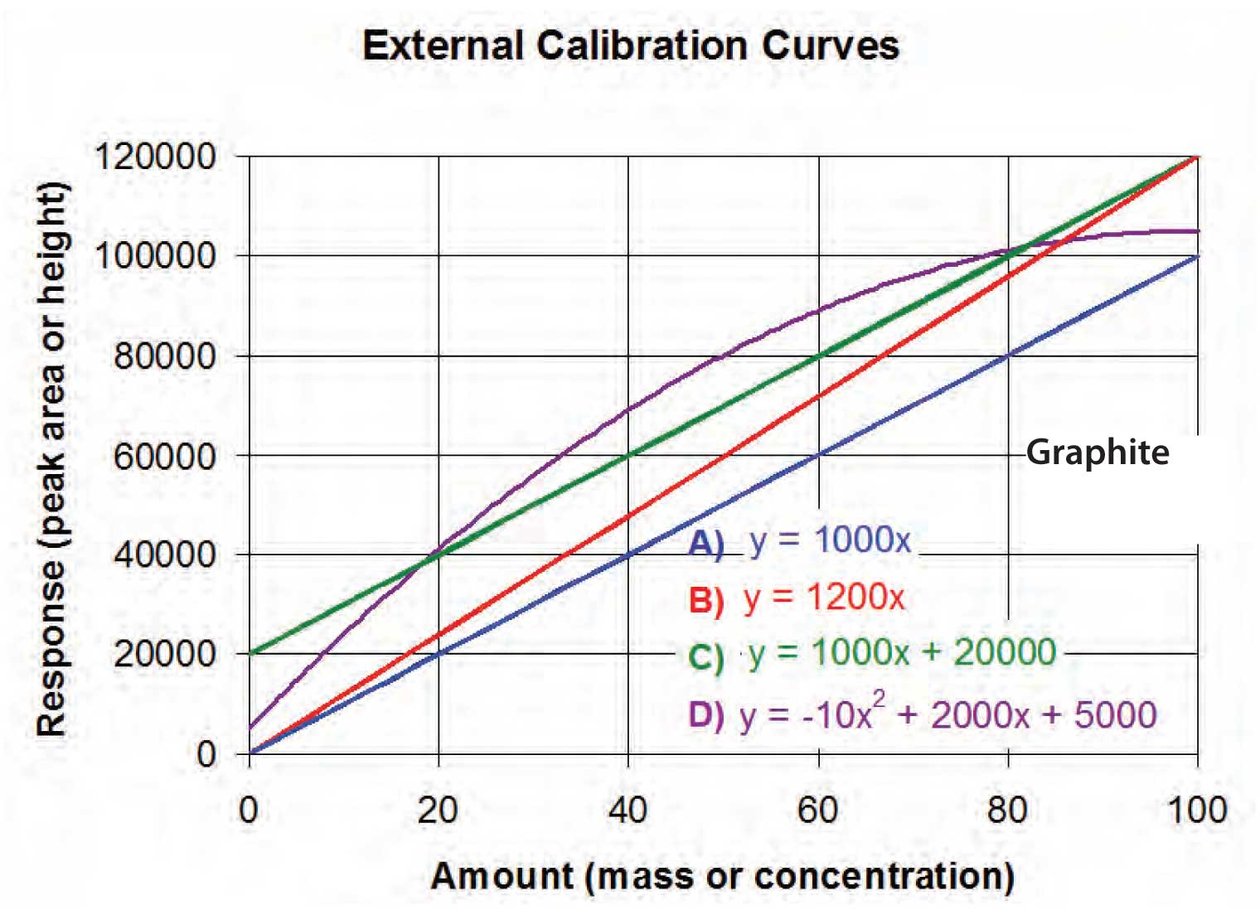
Calibration Curves вђ Part 1 Multipoint calibration is used for methods that cover a wide concentration range (for example, several orders of magnitude) and for methods where the calibration curve fits equation 1, but does not pass through the origin (b = 0). for example, multipoint calibration is used for methods to determine the concentration of a drug in plasma for a. Linearity is defined as. where y is the response (area), x is the concentration, m is the slope of the curve, and b is the y intercept. (even though the relationship is linear, we still call it a curve.) when the curve goes through the origin (x = 0, y = 0), b = 0, and the curve can be expressed as.
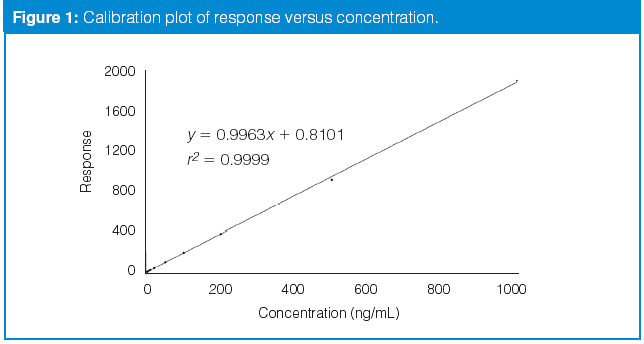
Calibration Curves Part 1 To B Or Not To B Chromatography Online In each case, the calibration curve benefits from weighting. for set 2, it appears that 1 x 0.5 should be adequate, whereas 1 x would be appropriate for set 3. little improvement is obtained with additional weighting for either of these data sets. it is a general observation that bioanalytical lc methods benefit from weighting up to 1 x 2 . Figure 5.4.1 shows the data in table 5.4.1 plotted as a normal calibration curve. although the data certainly appear to fall along a straight line, the actual calibration curve is not intuitively obvious. the process of determining the best equation for the calibration curve is called linear regression. 1.1 the correlation coefficient in part 1 of the tutorial, we saw how to use the trendline feature in excel to fit a straight line through calibration data and obtain both the equation of the best fit straight line and the correlation coefficient, r (sometimes displayed as r2). there are in fact various correlation coefficients, but the one we. Excel and openoffice calc versions. these are fill in the blanks spreadsheet templates for performing the calibration curve fitting and concentration calculations for analytical methods using the calibration curve method. all you have to do is to type in (or paste in) the concentrations of the standard solutions and their instrument readings (e.

Kalibrierungskurven Erstellung Und Nutzung Anvajo 1.1 the correlation coefficient in part 1 of the tutorial, we saw how to use the trendline feature in excel to fit a straight line through calibration data and obtain both the equation of the best fit straight line and the correlation coefficient, r (sometimes displayed as r2). there are in fact various correlation coefficients, but the one we. Excel and openoffice calc versions. these are fill in the blanks spreadsheet templates for performing the calibration curve fitting and concentration calculations for analytical methods using the calibration curve method. all you have to do is to type in (or paste in) the concentrations of the standard solutions and their instrument readings (e. A calibration curve plot showing limit of detection (lod), limit of quantification (loq), dynamic range, and limit of linearity (lol) in analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. [1]. When a calibration curve is a straight line, we represent it using the following mathematical equation. y = β0 β1x. where y is the analyte’s signal, sstd, and x is the analyte’s concentration, cstd. the constants β0 and β1 are, respectively, the calibration curve’s expected y intercept and its expected slope.

Calibration Curves A Factor V Calibration Curves Created Using A A calibration curve plot showing limit of detection (lod), limit of quantification (loq), dynamic range, and limit of linearity (lol) in analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. [1]. When a calibration curve is a straight line, we represent it using the following mathematical equation. y = β0 β1x. where y is the analyte’s signal, sstd, and x is the analyte’s concentration, cstd. the constants β0 and β1 are, respectively, the calibration curve’s expected y intercept and its expected slope.

The Calibration Curves Of The A D1 B D2 And C D3 Pyroelectric

Comments are closed.