Ccpoppy Cu Ccplaytime Cu Ccchapter Cu Cc2 Cuo Eoiotheeµ µeaapko Ith
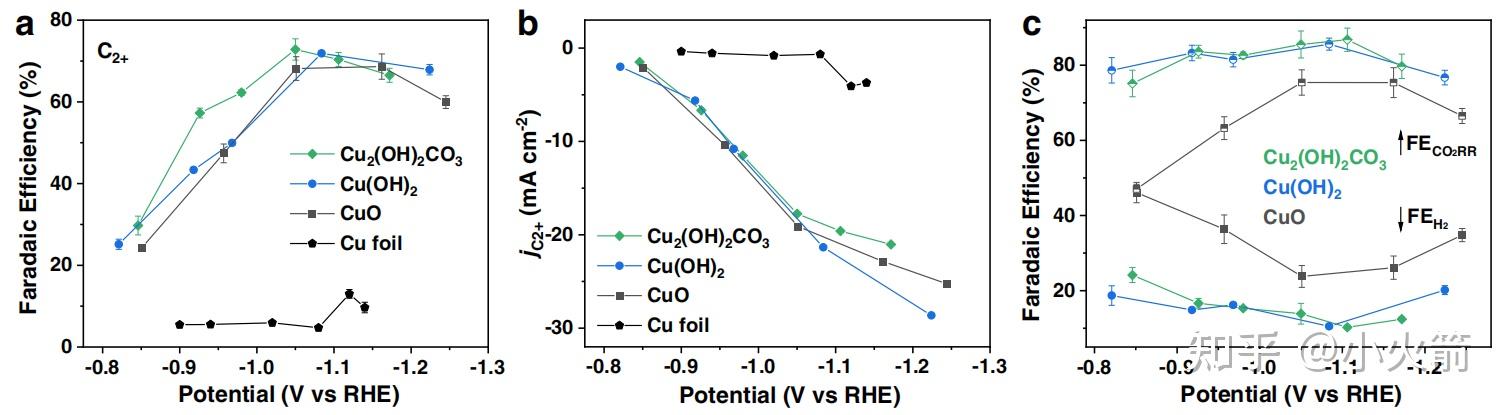
Cu 衍生物的结构演化和催化co2还原 Cu 被还原成0价 C2法拉第效率73 知乎 About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. In this video we'll balance the equation cuo c = cu co2 and provide the correct coefficients for each compound.to balance cuo c = cu co2 you'll need.

Cc2 4 7070 Welding Mold Type Cc2 70mm2 70mmві Abb Furse A n 2 o 5 b cu = c cuo d no now we write down algebraic equations to balance of each atom: n: a * 2 = d * 1 o: a * 5 = c * 1 d * 1 cu: b * 1 = c * 1 now we assign a=1 and solve the system of linear algebra equations: a * 2 = d a * 5 = c d b = c a = 1 solving this linear algebra system we arrive at: a = 1 b = 3 c = 3 d = 2. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. c12h22o11 24 cuo = 24 cu 12 co2 11 h2o. reactants. Cu2co3(oh)2 o2 = co2 cuo h2o is a combustion reaction where one mole of malachite [cu 2 co 3 (oh) 2] and zero moles of dioxygen [o 2] react to form one mole of carbon dioxide [co 2], two moles of copper(ii) oxide [cuo] and one mole of water [h 2 o]. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 cuo c = co2 2 cu. reactants. products.

Cu Sem 2 Education General а а їа а ќа а ѕа а їа ња ќа ћа ѕа ё а ња а ёа ѕа а а і Cc2 Ge Cu2co3(oh)2 o2 = co2 cuo h2o is a combustion reaction where one mole of malachite [cu 2 co 3 (oh) 2] and zero moles of dioxygen [o 2] react to form one mole of carbon dioxide [co 2], two moles of copper(ii) oxide [cuo] and one mole of water [h 2 o]. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 cuo c = co2 2 cu. reactants. products. The nanoscale cu–cuo interface can be easily controlled by adjusting the working parameters of the plasma. this paper mainly discusses the regulation of selectivity and activity of cu–cuo interface to co 2 rr. a series of catalysts with different cu–cuo interfaces were prepared by air plasma system and the relationship between the. Electrochemical conversion of carbon dioxide (co 2) into multi carbon fuels and chemical feedstocks is important but remains challenging.here, we report the stabilization of cu within a cuo–ceo 2 interface for efficient and selective electrocatalytic co 2 reduction to ethylene under ambient conditions.

Cu Political Science Honours Sem 1 Cc2 Suggestion 2023 а ёа ѕа а ђа а ѕа а ђ The nanoscale cu–cuo interface can be easily controlled by adjusting the working parameters of the plasma. this paper mainly discusses the regulation of selectivity and activity of cu–cuo interface to co 2 rr. a series of catalysts with different cu–cuo interfaces were prepared by air plasma system and the relationship between the. Electrochemical conversion of carbon dioxide (co 2) into multi carbon fuels and chemical feedstocks is important but remains challenging.here, we report the stabilization of cu within a cuo–ceo 2 interface for efficient and selective electrocatalytic co 2 reduction to ethylene under ambient conditions.

Comments are closed.