Ccrus Cu Cctilida Cu Cceng Cu Ccko Cu Ccp Cu Ccishlatiladigan Cu Cc100 Cu Suzlar Dadudi Dodydodydod
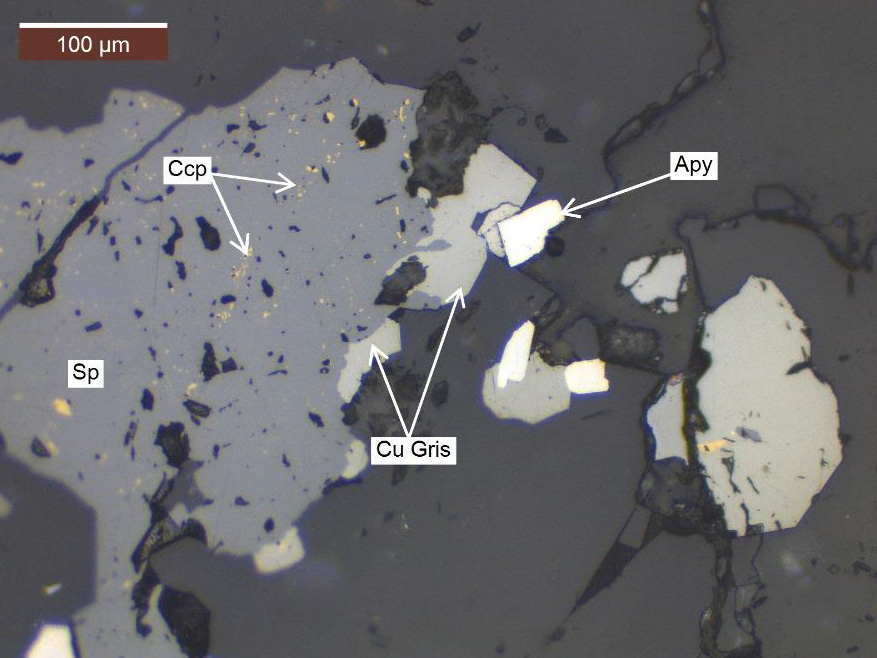
étude Minéralogique Cma Recently, syverson et al. (2021) determined the equilibrium cu isotope fractionation factor between chalcopyrite and aqueous cu(i) species at 350 °c, and found that the fractionation factor is small (Δ 65 cu ccp cu(aq) = –0.22 ± 0.16 ‰) and comparable in magnitude to those estimated via theoretical predictions (sherman, 2013, liu et al. Integration of a cvd co liner poses some unique challenges for both cu electrodeposition and cu cmp. the thin cu seed layer needs to be extremely smooth and continuous. rough cu seed layers (figure 2.7(a)) may contain thin, porous spots through which the highly acidic electrolytic cu plating bath can attack the underlying co. this results in.

Love Love Love Viral Short Trending Youtube There have been many reports concerning the reactivity of h 2 o 2 and cu, 13–16 cu etching properties, 17–26 properties of a surface reaction layer on cu films in a slurry, 27–32 planarization mechanism, 33–39 and the relation between dishing and polishing pressure. 40,41 as for aps slurry, to the best of our knowledge, there have been. Todd saliman is the 24th president of the university of colorado. a proud cu alum and lifelong coloradan, saliman was appointed president in july 2021. among saliman’s top priorities as president is connecting with communities across colorado to ensure cu meets the state’s evolving needs. under his leadership, cu’s outreach, engagement. The principle of ecmp is as shown in fig. 23.17, as follows: 1. chemical reaction when cu is oxidized by electricity cu → cu . 2. chemical reaction in which oxidized cu becomes cu (complex) cu →cu (complex) 3. mechanical reaction in which cu (complex) is removed by slurry and pad. Transmission rate and pattern density become the key factors affecting cu cmp edp curve at advanced node. different cu line pattern density determines the difference of cmp incoming topography. this incoming pattern loading requires cmp to leave enough process window to fix. this article provides the direction of cu cmp edp optimization for different pattern density products, and focuses on.

Comunicat Al Ccp Cu Privire La Aи A Zisul вђћbirou De Investigaи Iiвђќ вђ Crpo The principle of ecmp is as shown in fig. 23.17, as follows: 1. chemical reaction when cu is oxidized by electricity cu → cu . 2. chemical reaction in which oxidized cu becomes cu (complex) cu →cu (complex) 3. mechanical reaction in which cu (complex) is removed by slurry and pad. Transmission rate and pattern density become the key factors affecting cu cmp edp curve at advanced node. different cu line pattern density determines the difference of cmp incoming topography. this incoming pattern loading requires cmp to leave enough process window to fix. this article provides the direction of cu cmp edp optimization for different pattern density products, and focuses on. About two–thirds of all metals crystallize in closest packed arrays with coordination numbers of 12. metals that crystallize in an hcp structure include cd, co, li, mg, na, and zn, and metals that crystallize in a ccp structure include ag, al, ca, cu, ni, pb, and pt. O’reilly members experience books, live events, courses curated by job role, and more from o’reilly and nearly 200 top publishers. 12 group 11 (ib) [copper, silver and gold] copper, ag and au are known as coinage metals because they were used to make coins. all the metals …. selection from basic concepts of inorganic chemistry, 2nd.

Cu Prietena Mea Pe Zonдѓ Prezentarea Noastrдѓ Roblox Larisathegirl7 About two–thirds of all metals crystallize in closest packed arrays with coordination numbers of 12. metals that crystallize in an hcp structure include cd, co, li, mg, na, and zn, and metals that crystallize in a ccp structure include ag, al, ca, cu, ni, pb, and pt. O’reilly members experience books, live events, courses curated by job role, and more from o’reilly and nearly 200 top publishers. 12 group 11 (ib) [copper, silver and gold] copper, ag and au are known as coinage metals because they were used to make coins. all the metals …. selection from basic concepts of inorganic chemistry, 2nd.

Comments are closed.