Cctop Cu 10 Ccdangerous Cu Ccroads Cu Ccin The World Cu Si Seaseeseose Seo Sensiasii Seaseasiese Se

Underpotential Lithium Plating On Graphite Anodes Caused By Temperature Cú chulainn ( kuːˈkʌlɪn koo kul in[1][2] irish: [kuːˈxʊlˠɪn̠ʲ] ⓘ), is an irish warrior hero and demigod in the ulster cycle of irish mythology, as well as in scottish and manx folklore. [3] he is believed to be an incarnation of the irish god lugh, who is also his father. [4][5][6] his mother is the mortal deichtine, sister of. Electrocatalytic nitrate reduction reaction (no3–rr) has been considered a promising technology to produce ammonia but is inhibited by poor electrocatalytic performances. therefore, the development of efficient electrocatalysts and investigation of corresponding reaction mechanisms are critically important. herein, an efficient dendritic cu2o cu grown on cu foam (d cu2o cu cf) has been.
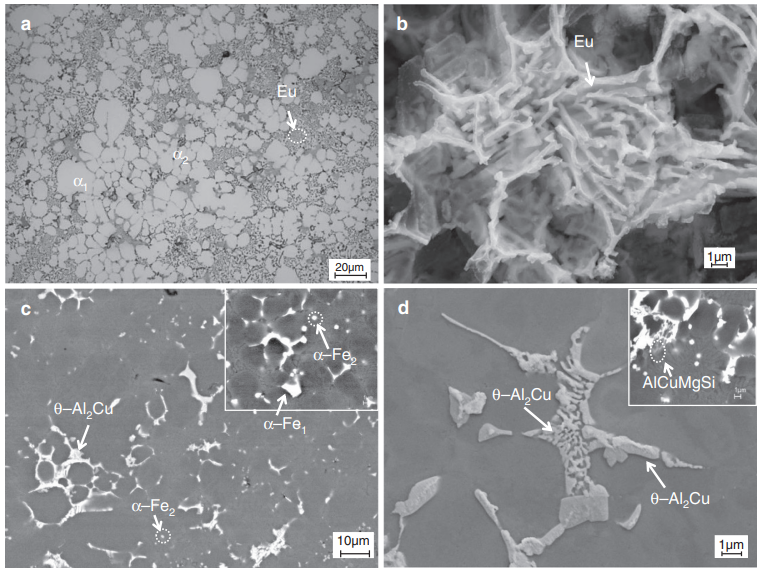
Fig 1 The As Cast Microstructure Of The Alвђ Siвђ Cu Alloy Containing 0 1 The beauty of avogadro's number is that it is always the same number, even for different elements. (4.5 ×1022 atoms cu)( 1 mol atoms cu 6.0221413× 1023 atoms cu) = 0.0747 mol atoms cu. if you don't get this result, check your parentheses. it should be solved with the following parentheses placements to avoid scaling the result by 1023:. The model cu electrodes were prepared by evaporating 25 nm of cu (~0.02 mg cm −2 loading) onto the microporous side of a gas diffusion electrode (gde) to form a thin layer of nanoparticles (fig. The schematic illustration in fig. 1 describes the synthesis process of cu co(oh) 2 and their transformation into cu cop. a facile hydrothermal process was used to prepare cobalt hydroxide porous nanoplates using polyethyleneimine (pei) as a chelating agent and copper cobalt hydroxide porous nanoplates by varying the concentration ratio of copper and cobalt nitrate (1:15, 1:10, 1:5, and 1:2. The copper cluster sizes slightly increased as the temperature during hydrogen reduction of the cuo ceo 2 precursor increased. on the cu 573 catalyst, cu clusters 1.1 nm wide and 0.44 nm thick.

р рёр рісђр рјрјр сѓрѕсѓс рѕсџрѕрёсџ сѓрёсѓс рµрјс Cu Si The schematic illustration in fig. 1 describes the synthesis process of cu co(oh) 2 and their transformation into cu cop. a facile hydrothermal process was used to prepare cobalt hydroxide porous nanoplates using polyethyleneimine (pei) as a chelating agent and copper cobalt hydroxide porous nanoplates by varying the concentration ratio of copper and cobalt nitrate (1:15, 1:10, 1:5, and 1:2. The copper cluster sizes slightly increased as the temperature during hydrogen reduction of the cuo ceo 2 precursor increased. on the cu 573 catalyst, cu clusters 1.1 nm wide and 0.44 nm thick. A new cu si al o h reactive reaxff force field was developed and used in molecular dynamics simulations of the hydration of cu exchanged ssz 13 catalyst. it was observed that at temperatures close to room temperarture, all cu cations, including those at the faces of the double 6 member rings (6mrs), become fully hydrated and detach from the framework. the hydrated cu cations can diffuse. Experimental isothermal sections of the ternary phase diagram al–cu–si at 600 °c and 800 °c. o. zobač a. kroupa k. richter. materials science. journal of materials science. 2020. the phase diagram of the al–cu–si ternary system was investigated experimentally at the temperatures 600 °c and 800 °c. the current study was designed to.

Comments are closed.