Cctradingviewucaoiuo Nµyoth Uthon Cuthc Oe Oiu Cco Nµyoo Ioio Cuo Tha Ccooooo Ooooo Cu
Frontiers Membrane Free Electrocatalysis Of Co2 To C2 On Cuo Ceo2 Crude oil prices & gas price charts. oil price charts for brent crude, wti & oil futures. energy news covering oil, petroleum, natural gas and investment advice. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). the ‘ii’ in its name signifies that the copper ion in this compound has a 2 oxidation state. this is a result of copper losing two of its electrons to oxygen, which is more electronegative. the compound’s structure is characterized by a.

Photocatalytic C C Coupling From Carbon Dioxide Reduction On Copper Notes. copper can be produced from many different raw materials, the main being black tenorite (cuo), deep red cuprite (cu 2 o), bright green malachite (cuco 3.cu (oh)2), and bright blue azurite (2cuco 3.cu (oh)2). under normal oxidizing conditions the cuo molecule remains unchanged and produces clear green colors in oxidation glazes. Copper oxide (cuo) is a highly insoluble thermally stable copper source suitable for glass, optic and ceramic applications. copper oxide is a black solid known as tenorite in mineral form, it can be formed by heating copper in the presence of oxygen. oxide compounds are not conductive to electricity. however, certain perovskite structured. Cupric oxide, or copper (ii) oxide, is an inorganic compound with the chemical formula cuo. cupric oxide is used as a precursor in many copper containing products such as wood preservatives and ceramics. cupric oxide may be found in over the counter vitamin mineral supplements as a source of [db09130]. the mean daily dietary intake of copper. Copper (ii) oxide is made of two ions; the cupric ion, cu 2, and the oxygen ion, o 2. one cupric ion transfers its two electrons to the oxygen ion. cu 2 o 2 → cuo. one cupric ion is required.
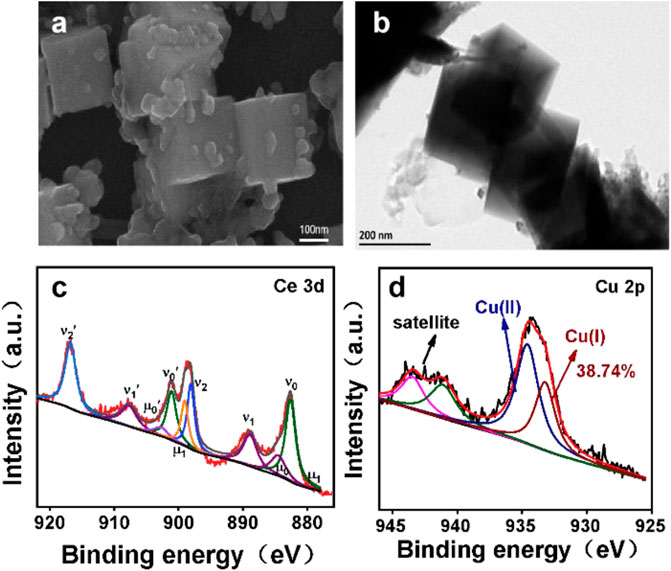
Coatings Free Full Text In Situ Synthesis Of Cuo Cu2o Nanoparticle Cupric oxide, or copper (ii) oxide, is an inorganic compound with the chemical formula cuo. cupric oxide is used as a precursor in many copper containing products such as wood preservatives and ceramics. cupric oxide may be found in over the counter vitamin mineral supplements as a source of [db09130]. the mean daily dietary intake of copper. Copper (ii) oxide is made of two ions; the cupric ion, cu 2, and the oxygen ion, o 2. one cupric ion transfers its two electrons to the oxygen ion. cu 2 o 2 → cuo. one cupric ion is required. The normalized thermal conductivity of cu, cuo, and mwnt nanofluids as a function of the volume fraction. (a) the normalized thermal conductivity of cuo ethylene glycol nanofluids as a function of. Cuprous oxide is more toxic for bacteria than cupric oxide because of the presence of one oxygen atom in cuprous oxide and two oxygen atoms in cupric oxide. a cuprous oxide is an active form of copper (i) oxide whereas cupric oxide is a more stable form of copper (i) oxide. cuprous oxide is more toxic because it can oxidise the phosphate groups of cellular components which are essential for.

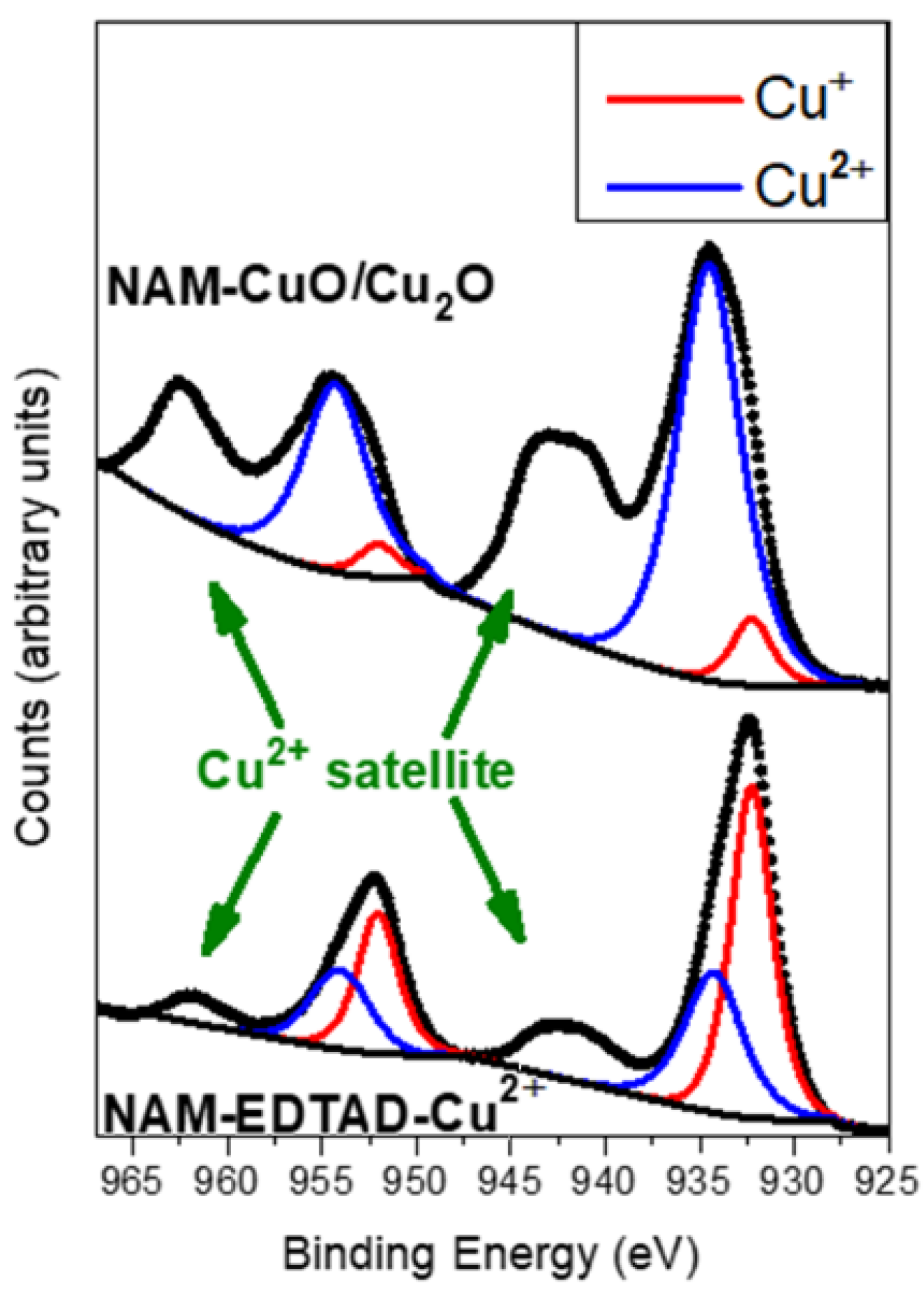
A Cuo Unit Cell B Cu 4 O 4 Cluster And C Cuo 1 1 1 Slab With The normalized thermal conductivity of cu, cuo, and mwnt nanofluids as a function of the volume fraction. (a) the normalized thermal conductivity of cuo ethylene glycol nanofluids as a function of. Cuprous oxide is more toxic for bacteria than cupric oxide because of the presence of one oxygen atom in cuprous oxide and two oxygen atoms in cupric oxide. a cuprous oxide is an active form of copper (i) oxide whereas cupric oxide is a more stable form of copper (i) oxide. cuprous oxide is more toxic because it can oxidise the phosphate groups of cellular components which are essential for.

Comments are closed.