Chemistry Class 12 Ncert Solutions Chapter 3 Electrochemistry
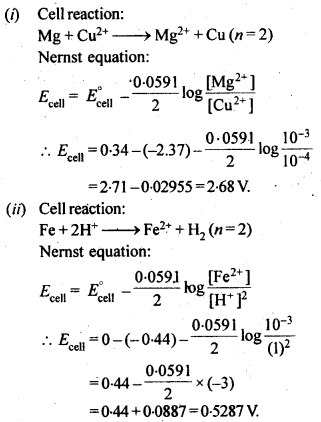
Ncert Solutions For Class 12 Chemistry Chapter 3 Electr Ncert textbook questions solved. 3.1. how would you determine the standard electrode potential of the system mg 2 1 mg? ans: a cell will be set up consisting of mg mgso 4 (1 m) as one electrode and standard hydrogen electrode pt, h, (1 atm)h (l m) as second electrode, measure the emf of the cell and also note the direction of deflection in the voltmeter. Class 12 chemistry ncert solutions for chapter 3 – electrochemistry have been designed to help the students prepare well and score good marks in the cbse class 12 chemistry exam. further, the solutions consist of well thought and structured questions, along with detailed explanations, to help students learn and remember concepts easily.

Ncert Solutions For Class 12 Chemistry Chapter 3 Electr Three substances that can do so are f 2, cl 2, and o 2. ncert solutions for class 12 chemistry chapter 4. question 4. calculate the potential of hydrogen electrode in contact with a solution whose ph is 10. solution : for hydrogen electrode, , it is given that ph = 10. ∴ [h ] = 10 −10 m. Calculate the potential of hydrogen electrode in contact with a solution whose ph is 10. solution: question 5. calculate the emf of the cell in which the following reaction takes place : ni (s) 2ag (0.002 m) → ni 2 (0.160 m) 2ag (s) given that e°cell = 1.05 v. solution: question 6. The detailed, step by step solutions will help you understand the concepts better and clarify any confusion. ncert solutions for mathematics chemistry class 12 cbse, karnataka board puc 3 (electrochemistry) include all questions with answers and detailed explanations. this will clear students' doubts about questions and improve their. Ncert solutions for class 12 chemistry chapter 3 electrochemistry the reaction with a higher value of e0 takes place at the cathode. therefore, deposition of silver will take place at the cathode. at anode: (iii) at the cathode, the following reduction reaction occurs to produce h 2 gas. at the anode, the following processes are possible.

Ncert Solutions For Chemistry Class 12 Chapter 3 Electr The detailed, step by step solutions will help you understand the concepts better and clarify any confusion. ncert solutions for mathematics chemistry class 12 cbse, karnataka board puc 3 (electrochemistry) include all questions with answers and detailed explanations. this will clear students' doubts about questions and improve their. Ncert solutions for class 12 chemistry chapter 3 electrochemistry the reaction with a higher value of e0 takes place at the cathode. therefore, deposition of silver will take place at the cathode. at anode: (iii) at the cathode, the following reduction reaction occurs to produce h 2 gas. at the anode, the following processes are possible. Ncert solutions for class 12 chemistry chapter 3 – electrochemistry q 3.1: arrange the following metals in the order in which they displace each other from the solution of their salts. al, cu, fe, mg and zn. answer: according to their reactivity, the given metals replace the others from their salt solutions in the said order: mg, al, zn, fe. Electrochemistry class 12 ncert notes. important points and formulas of ncert class 12 chemistry chapter 3 electrochemistry . 1. conductance (g) is the reciprocal of resistance (r) and specific conductance or conductivity (k) is inverse of resistivity. 2. l a is called the cell constant of conductivity cell.
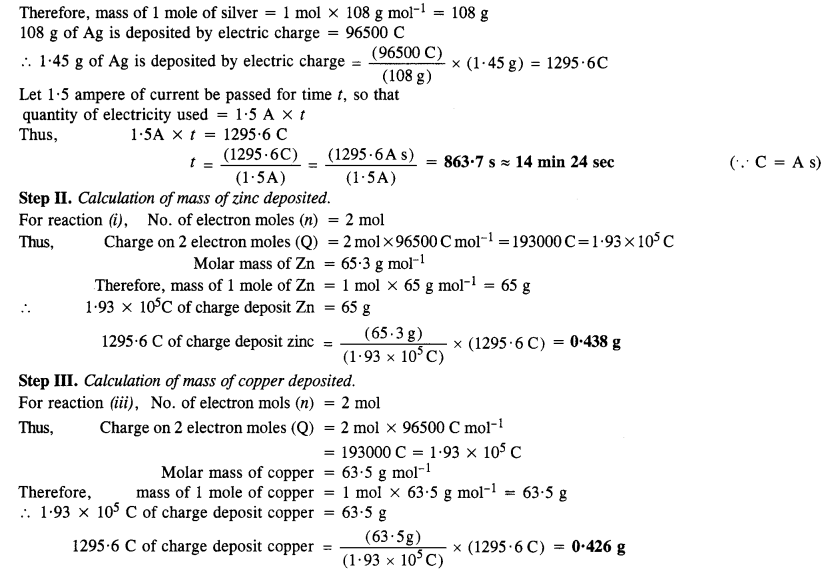
Ncert Solutions For Class 12 Chemistry Chapter 3 Electr Ncert solutions for class 12 chemistry chapter 3 – electrochemistry q 3.1: arrange the following metals in the order in which they displace each other from the solution of their salts. al, cu, fe, mg and zn. answer: according to their reactivity, the given metals replace the others from their salt solutions in the said order: mg, al, zn, fe. Electrochemistry class 12 ncert notes. important points and formulas of ncert class 12 chemistry chapter 3 electrochemistry . 1. conductance (g) is the reciprocal of resistance (r) and specific conductance or conductivity (k) is inverse of resistivity. 2. l a is called the cell constant of conductivity cell.

Chemistry Class 12 Ncert Solutions Chapter 3 Electrochemistry вђ Class

Comments are closed.