Chemistry Practical Salt Analysis Complete Acid Radical Analysis

Chemistry Practical Salt Analysis Complete Acid Radical Analysis Step 1: obtain the inorganic salt whose cation and anion you must identify. step 2: conduct preliminary tests for the anion group wise until you obtain a positive result. anions and cations are classified into groups that share the same group reagent and therefore, have similar preliminary tests. Hii am dr p k singhal welcome you all to my channel chemistry pandit – singhal sirdear students this video will help you to understand about the salt.

Chemistry Practical Salt Analysis Acidic Radicals Chemistry Iq The document describes four experiments to identify the basic and acidic radicals present in different salts through a series of chemical tests. in experiment 1, the salt was found to contain na as the basic radical and cl as the acidic radical based on its solubility, flame test, and confirmatory tests for anions. experiment 2 identified the salt as containing nh4 as the basic radical and. Nitrite (no2) acid radical test | anion test | salt analysis |chemistry practicalclass 11 and 12 practical chemistry @prakashchemistrypractical. Hi learners,thank you so much for watching the top coaching session. this video takes you through the proper technique for setting up and performing. 2. o it is primary standard, hence is solution can be prepared by direct weighing. molecular weight of mohr’s salt : 56 32 4×16 2(14 4) 32 4×16 6×18 = 392 g. thus to prepare 1000 ml of 1m mohr’s salt solution, 392 g of mohr’s salt is needed. to prepare 250ml of 1 m mohr’s salt.
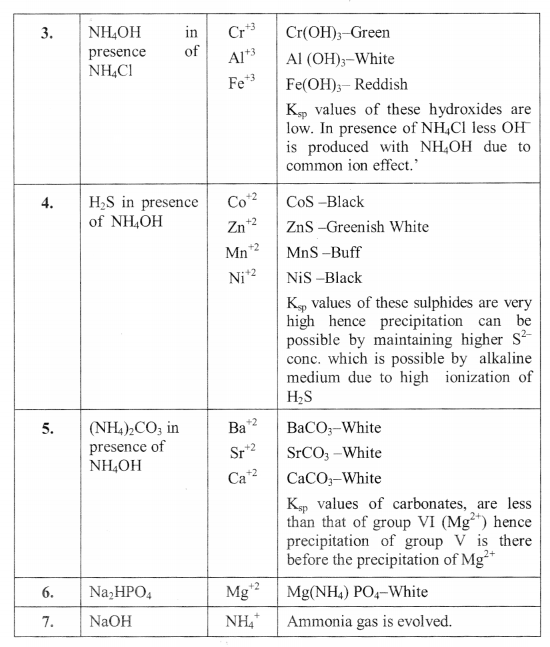
Salt Analysis Formulas Complete List Formulae Sheet For Salt Analysis Hi learners,thank you so much for watching the top coaching session. this video takes you through the proper technique for setting up and performing. 2. o it is primary standard, hence is solution can be prepared by direct weighing. molecular weight of mohr’s salt : 56 32 4×16 2(14 4) 32 4×16 6×18 = 392 g. thus to prepare 1000 ml of 1m mohr’s salt solution, 392 g of mohr’s salt is needed. to prepare 250ml of 1 m mohr’s salt. Add freshly prepared ferrous sulfate without shaking on the sides of the test tube. at the junction of the two solutions, a dark brown ring is formed. 7. cl ‒ (chloride anion) in a test tube, take 0.1 g of salt, add a pinch of manganese dioxide and 3 4 drops of conc. acid with sulfuric acid. The salt by balancing the charges of the cation and anion. for example, if your cation is fe3 and your anion is cl , the chemical formula of the salt will be fecl 3. note: you can also identify the cation first and then move on to identifying the anion. salt analysis answer format (sample) a sample answer format for salt analysis is provided.

Comments are closed.