Clinical Trial Definitions
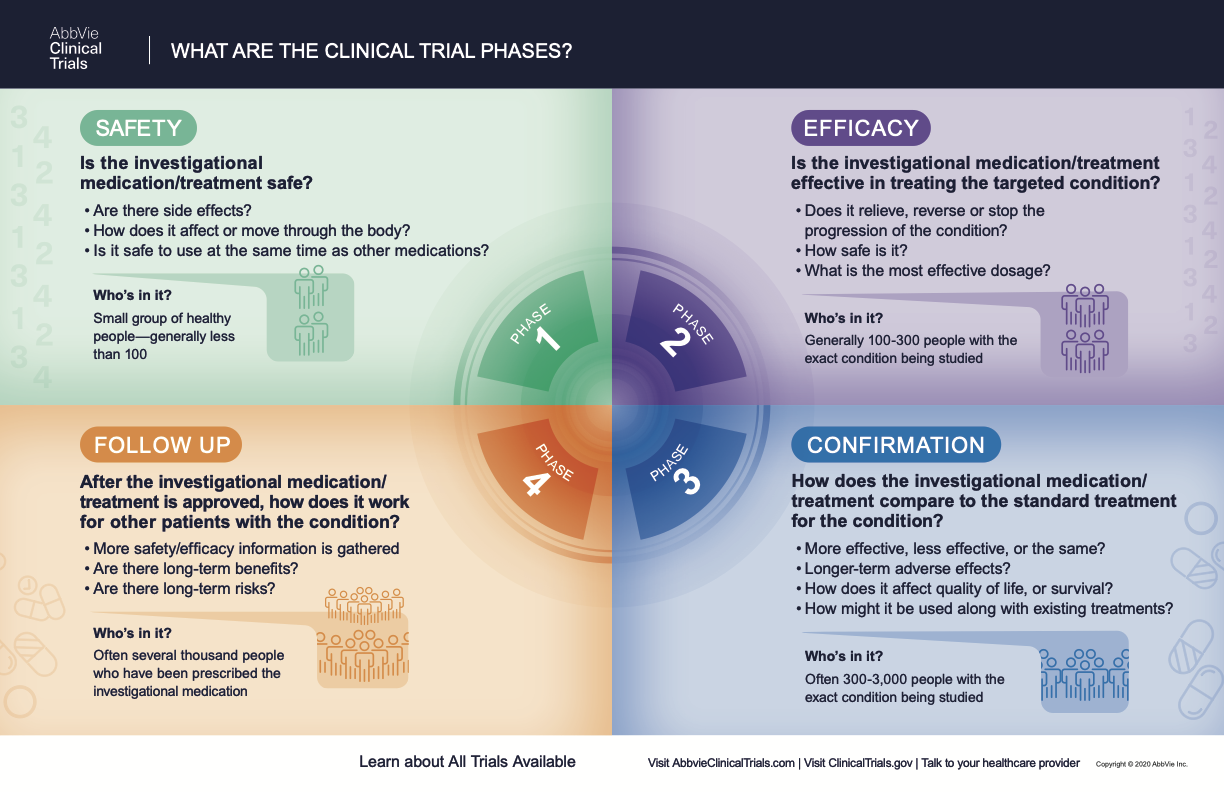
What Are The Phases Of Clinical Trials Clinical Trial Phases Learn how to identify a clinical trial according to nih's revised definition, which involves human subjects, interventions, and health related outcomes. use the decision tool, case studies, faqs, and decision tree to clarify the definition. Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current.

Clinical Trials Gene Vision Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes. people volunteer to take part in clinical trials to test medical interventions including drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioural treatments and preventive care. Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. these trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people. A clinical trial participant receives an injection. clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices. Clinical trial: [noun] a scientifically controlled study of the safety and effectiveness of a therapeutic agent (such as a drug or vaccine) using consenting human subjects.
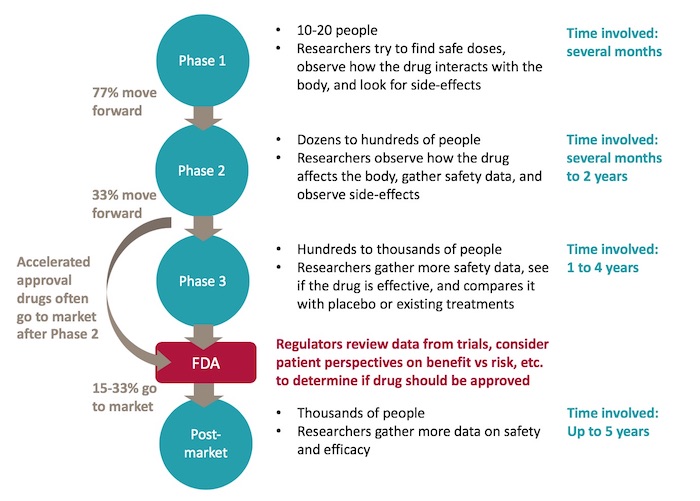
Clinical Trials Fshd Society A clinical trial participant receives an injection. clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices. Clinical trial: [noun] a scientifically controlled study of the safety and effectiveness of a therapeutic agent (such as a drug or vaccine) using consenting human subjects. Facebook email print. clinical trials are medical research studies in which people participate as volunteers. they help researchers better understand the normal biological processes, learn more about diseases and conditions, and develop new treatments and medications. The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. people who take part in clinical research make it possible for this to occur. the path to finding out if a new drug is safe or effective is to test it on patients in clinical trials.

Pdf Clinical Trial Design Principles And Endpoint Definitions Facebook email print. clinical trials are medical research studies in which people participate as volunteers. they help researchers better understand the normal biological processes, learn more about diseases and conditions, and develop new treatments and medications. The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. people who take part in clinical research make it possible for this to occur. the path to finding out if a new drug is safe or effective is to test it on patients in clinical trials.
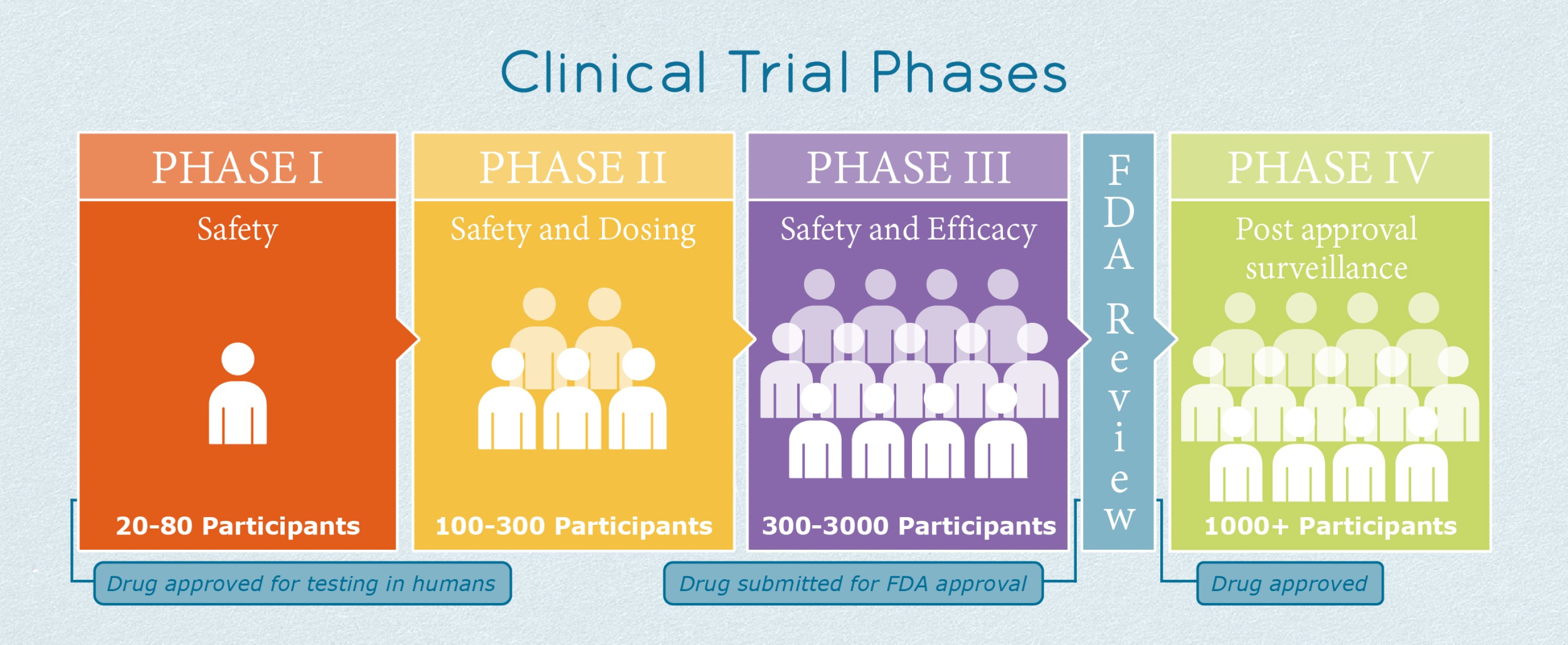
Phase 4 Clinical Trial

Comments are closed.