Clinical Trial Glossary Phases Of Clinical Research Clinical Trial
Clinical Trial Phases Diagram Glossary of common terms. Clinicaltrials.gov glossary terms.

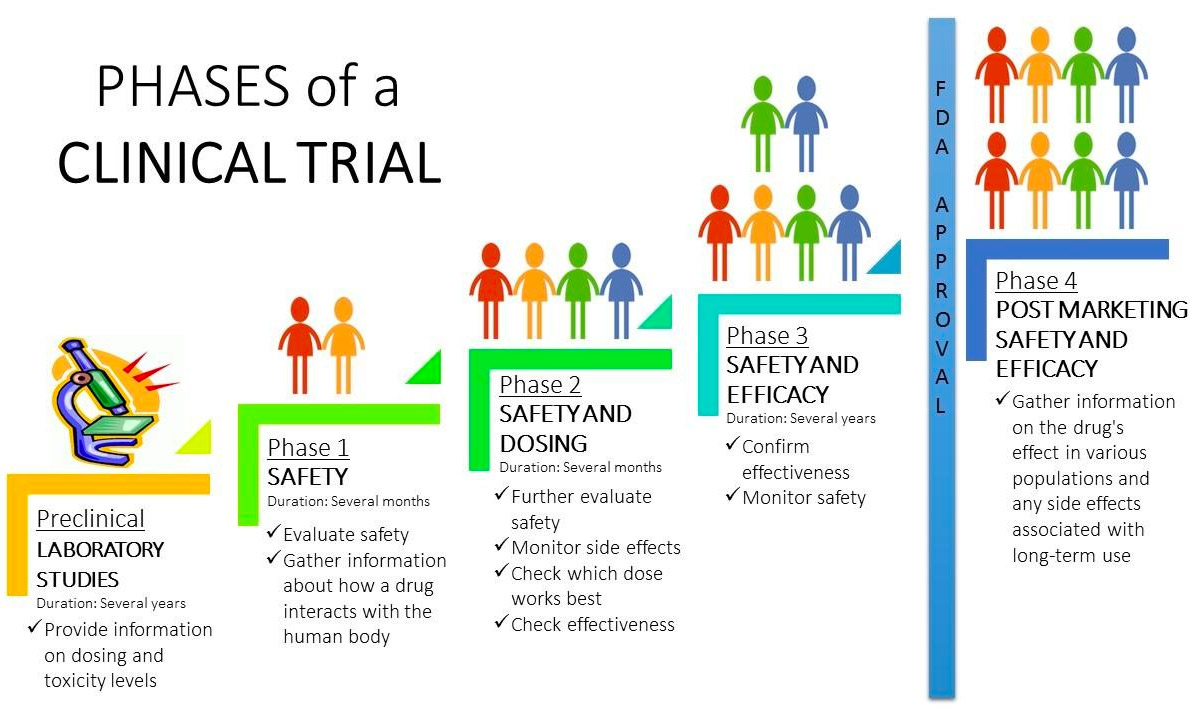
Phase Clinical Research Glossary Clinical trials are conducted in phases that take the trial through each stage before the u.s. food and drug administration (fda) approves it. at least three phases are required before a new medication can be approved by the fda. phase i trials: these first studies in people evaluate how a new drug should be given (by mouth, injected into the. Phase 1 — a small study, typically using healthy volunteers, that looks at the safety and dosing of a drug. phase 2 — a larger study of people with the condition or disease being studied who receive either a placebo or intervention, used to determine safety and efficacy. phase 3 — a much larger study of people with the condition or. A clinical trial is a research study designed to learn how our bodies respond to investigational medicines or other investigational treatments. during the clinical trial, participants are assigned to get a treatment or sometimes, no treatment. the purpose of a trial is usually to find ways to prevent, diagnose, or treat a disease or other. Clinical research or study coordinator (crc) – an individual that handles the administrative and day to day responsibilities of a clinical trial and acts as a liaison for the clinical site. this person may collect the data or review it before it is entered into a study database. clinical research – nih defines clinical research as: patient.

Comments are closed.