Clinical Trial Investigator What Do Clinical Trial Investigators And
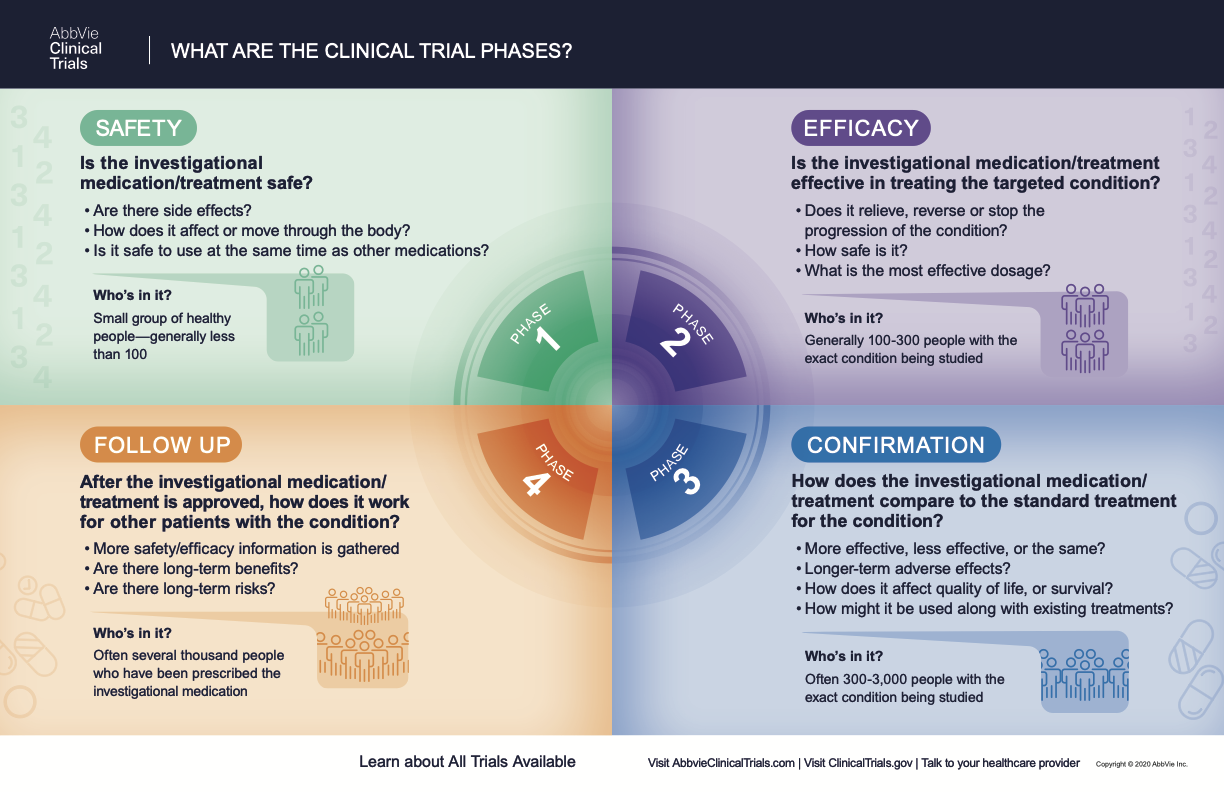
What Are The Phases Of Clinical Trials Clinical Trial Phases For purposes of [21 cfr part 54], “clinical investigator” means a “listed or identified investigator or sub investigator who is directly involved in the treatment or evaluation of research subjects,” including the spouse and each dependent child of the investigator or sub investigator. (see 21 cfr § 54.2 (d).). Investigator: ich e6 (r2) good clinical practice. 4.1 investigator’s qualifications and agreements. 4.1.1 the investigator (s) should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial, should meet all the qualifications specified by the applicable regulatory requirement (s), and.

Clinical Trial Investigator What Do Clinical Trial Investigators And If the fda rules against the investigator, the individual may be temporarily or permanently banned from participating in trials of investigational drugs or medical devices. debarments are listed on the fda website. 24. the ketek case provides multiple examples of egregious clinical trial violations and problems at the fda. Here are the steps to follow for how to become a clinical trial investigator: 1. become a physician. exact requirements to become a clinical trial investigator vary based on the agency or company conducting the trial. however, depending on the goal of the trial, most groups require you to be a physician, a psychiatrist, a nurse practitioner or. 312.64 investigator reports. (a) progress reports. the investigator shall furnish all reports to the sponsor of the drug who is responsible for collecting and evaluating the results obtained. the. Investigators must be diligent throughout the conduct of a clinical trial: while designing the protocol, when deciding which trials to conduct, during the performance of the study, and after conclusion of the study ( fig 1 ). although there are multiple regulatory safeguards designed to ensure the ethical conduct of research, it is ultimately.

How To Apply For A Clinical Trial Miami Clinical Research 312.64 investigator reports. (a) progress reports. the investigator shall furnish all reports to the sponsor of the drug who is responsible for collecting and evaluating the results obtained. the. Investigators must be diligent throughout the conduct of a clinical trial: while designing the protocol, when deciding which trials to conduct, during the performance of the study, and after conclusion of the study ( fig 1 ). although there are multiple regulatory safeguards designed to ensure the ethical conduct of research, it is ultimately. Become a principal or sub investigator (pi, sub i) we seek talented, experienced physicians (md, do) and advanced practitioners (np, pa, pharmd, phds) licensed to practice medicine in texas and new mexico, to become clinical trial investigators! investigator benefits. offer cutting edge, life saving treatment options. earn additional revenue. 3 the clinical trial site and the investigator 3.1 definition and purpose of a clinical trial site 8 3.2 activities of a clinical trial site 8 3.2.1 protocol development scientific review and biostatistical consultation 8.

Are You Eligible For A Clinical Trial Neuroendocrine Cancer Australia Become a principal or sub investigator (pi, sub i) we seek talented, experienced physicians (md, do) and advanced practitioners (np, pa, pharmd, phds) licensed to practice medicine in texas and new mexico, to become clinical trial investigators! investigator benefits. offer cutting edge, life saving treatment options. earn additional revenue. 3 the clinical trial site and the investigator 3.1 definition and purpose of a clinical trial site 8 3.2 activities of a clinical trial site 8 3.2.1 protocol development scientific review and biostatistical consultation 8.

Comments are closed.