Clinical Trial Phases 1 2 3 4 Find Fda Clinical Trial P
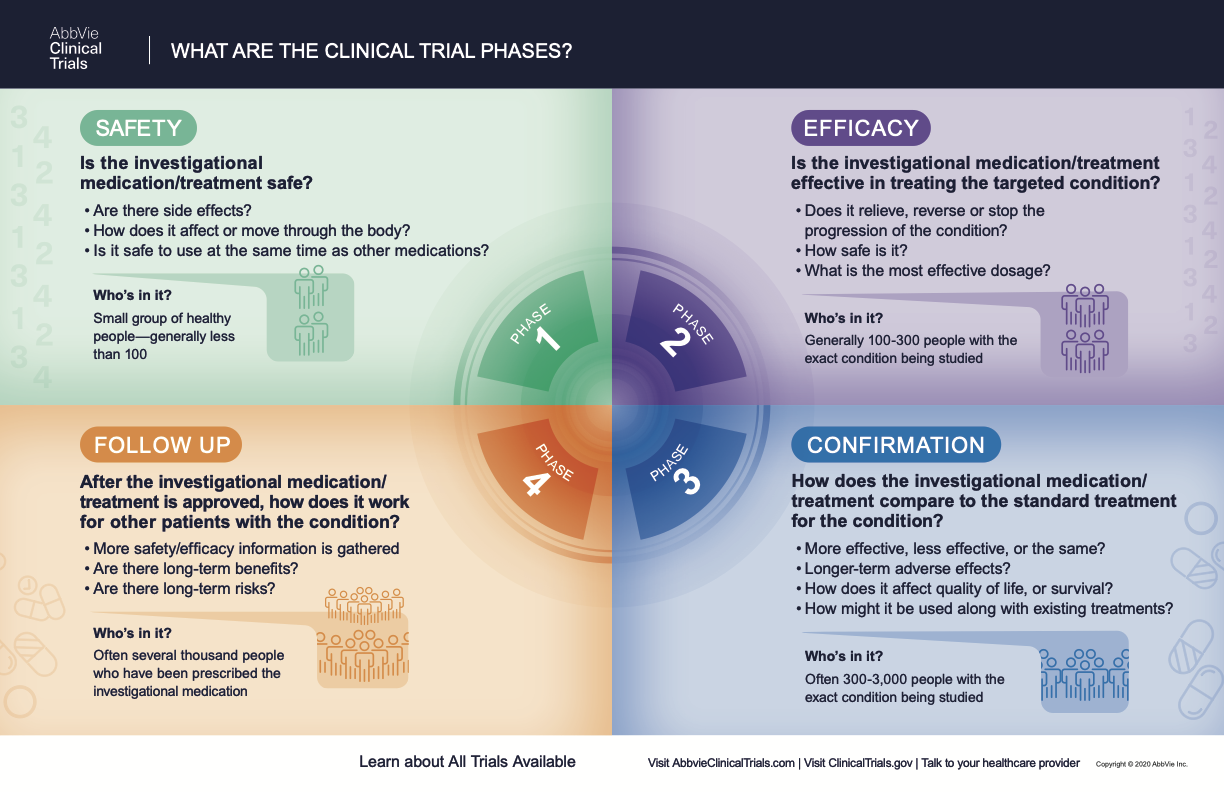
What Are The Phases Of Clinical Trials Clinical Trial Phases When phase 3 clinical trials (or sometimes phase 2 trials) show a new drug is more effective and safer than the current standard treatment, a new drug application (nda) is submitted to the food and drug administration (fda) for approval. the nda, which includes data from all the pre clinical and clinical studies, is reviewed by the fda. Watch this video to learn about the three phases of clinical trials. clinical research phase studies. phase 1. study participants: 20 to 100 healthy volunteers or people with the disease condition.
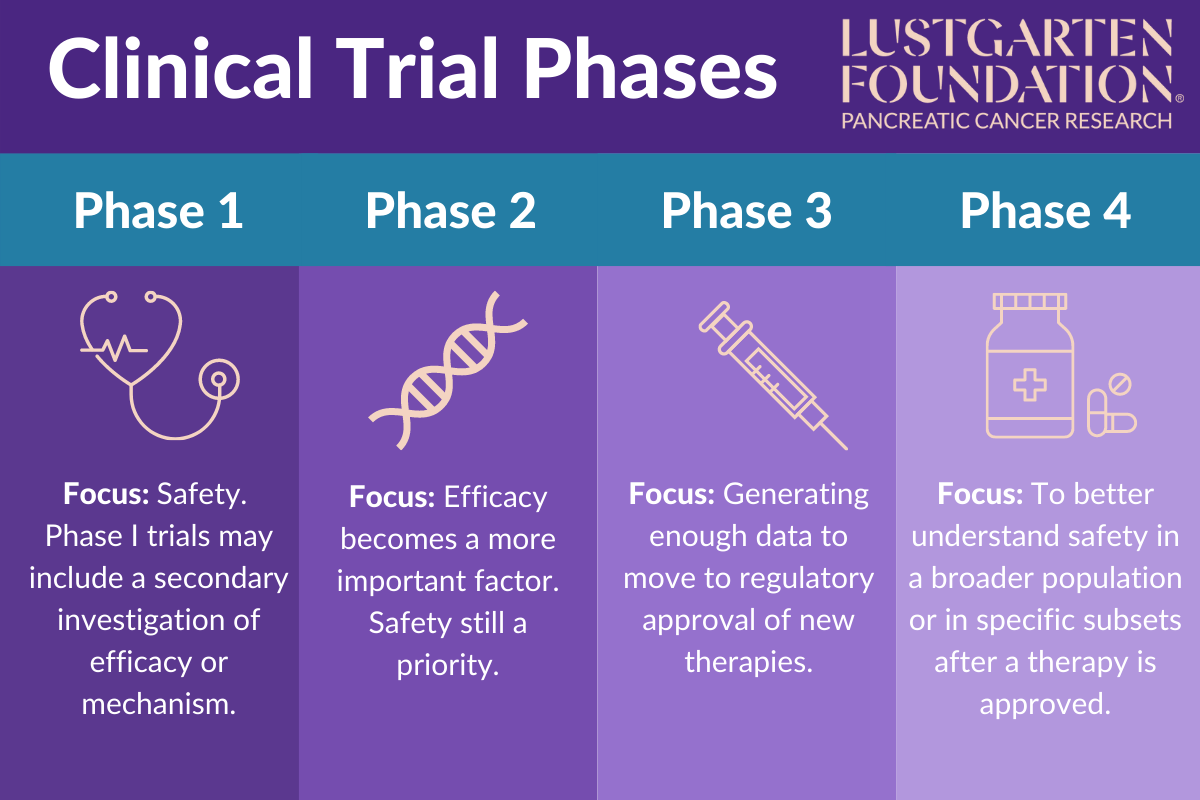
Clinical Trial Phases Key points of phase iii clinical trials. most phase iii clinical trials include a large number of patients, at least several hundred. these studies are often done in many places across the country (or even around the world) at the same time. phase iii clinical trials are more likely to be offered in local community hospitals and doctor's offices. Learn about clinical trials for people with cancer. aids clinical trials and information services (actis) or call 1–800–trials–a (1–800–874–2572). locate clinical trials for people. The four phases of clinical trials. clinical trial phases. the process of learning about and developing an investigational medicine is divided into four phases. at first, very few people receive the medicine being studied. the number of people participating in clinical studies grows along with our understanding of the investigational medicine. The fda usually requires a phase iii clinical trial before approving a new medication. due to the larger number of participants and longer duration or phase iii, rare and long term side effects.

Comments are closed.