Clinical Trials Part 3 Phases Of Clinical Trials And What Happen
Historical Clinical Trials вђ Lг Dia Andrг Clinical trials happen in several phases during which different questions are asked. each phase builds on the results of previous phases. keep reading to learn more about what happens during each. There are benefits and risks to taking part in each phase of a clinical trial. although there are clinical trials for devices as well as other diseases and treatments, drugs for cancer patients are used in the examples of clinical trial phases described here. phase 0 clinical trials: exploring if and how a new drug may work.

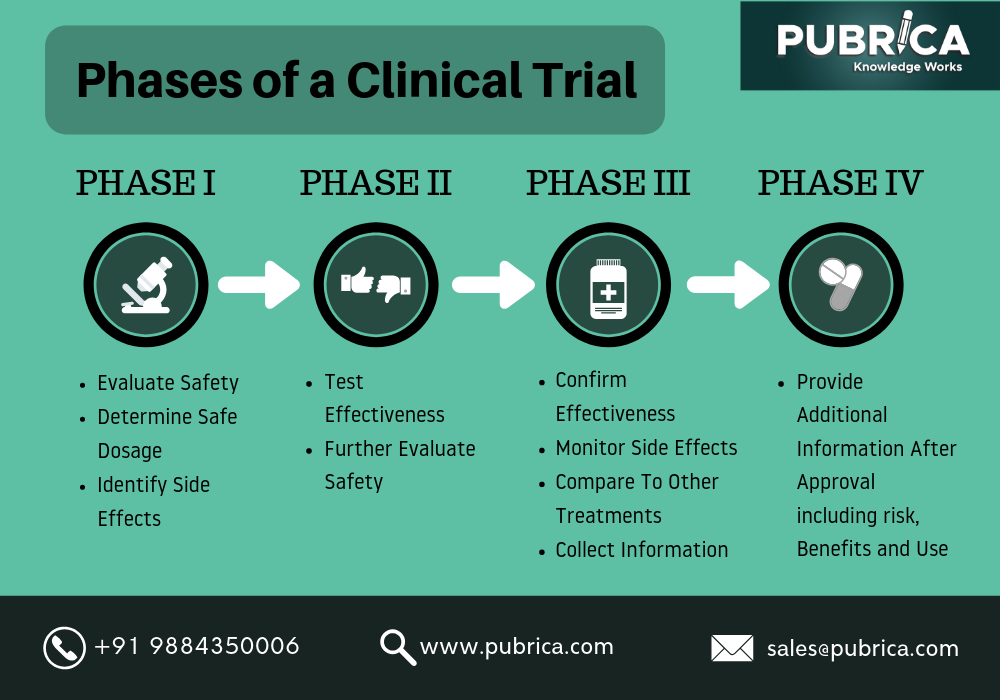
Cancer Treatment Clinical Trials Doctorvisit Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Watch this video to learn about the three phases of clinical trials. clinical research phase studies. phase 1. study participants: 20 to 100 healthy volunteers or people with the disease condition. Phase 3 clinical trials compare new treatments with standard treatments. they also can compare the response to a new treatment with how a group of patients responded to a different treatment. in phase 3 trials, researchers often try to see which treatment is safer and works best. there are usually between 300 and 3,000 people in a phase 3 study. The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects.

What Are Clinical Trials Debra Ireland Phase 3 clinical trials compare new treatments with standard treatments. they also can compare the response to a new treatment with how a group of patients responded to a different treatment. in phase 3 trials, researchers often try to see which treatment is safer and works best. there are usually between 300 and 3,000 people in a phase 3 study. The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects. Phases of clinical trials. clinical trials to test new cancer treatments involve a series of steps called phases. if a new treatment is successful in one phase, it will proceed to further testing in the next phase. during the early phases (phases 1 and 2), researchers figure out whether a new treatment is safe, its side effects, and its best dose. In phase 3 trials, researchers try to find out if a treatment works in a large number of people, usually about 1,000 to 5,000 participants who have the health condition the treatment is intended to treat. in vaccine trials, the participants may be healthy or have diseases or conditions. phase 3 trials may happen in a doctor’s office, a clinic.
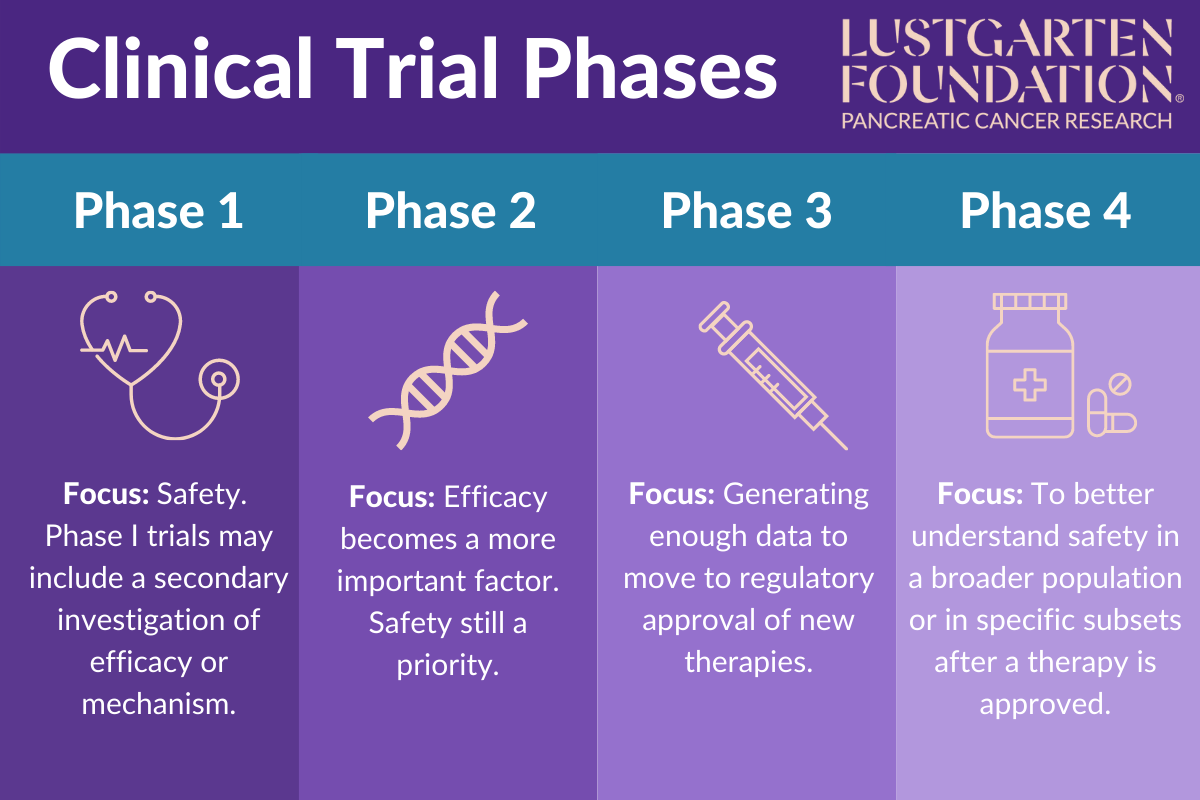
Clinical Trial Phases Phases of clinical trials. clinical trials to test new cancer treatments involve a series of steps called phases. if a new treatment is successful in one phase, it will proceed to further testing in the next phase. during the early phases (phases 1 and 2), researchers figure out whether a new treatment is safe, its side effects, and its best dose. In phase 3 trials, researchers try to find out if a treatment works in a large number of people, usually about 1,000 to 5,000 participants who have the health condition the treatment is intended to treat. in vaccine trials, the participants may be healthy or have diseases or conditions. phase 3 trials may happen in a doctor’s office, a clinic.

All About Cancer Clinical Trials Trial Safety Measures Masonic

Comments are closed.