Constitutional Isomers Of C5h10o Aldehyde Ketone Dr K
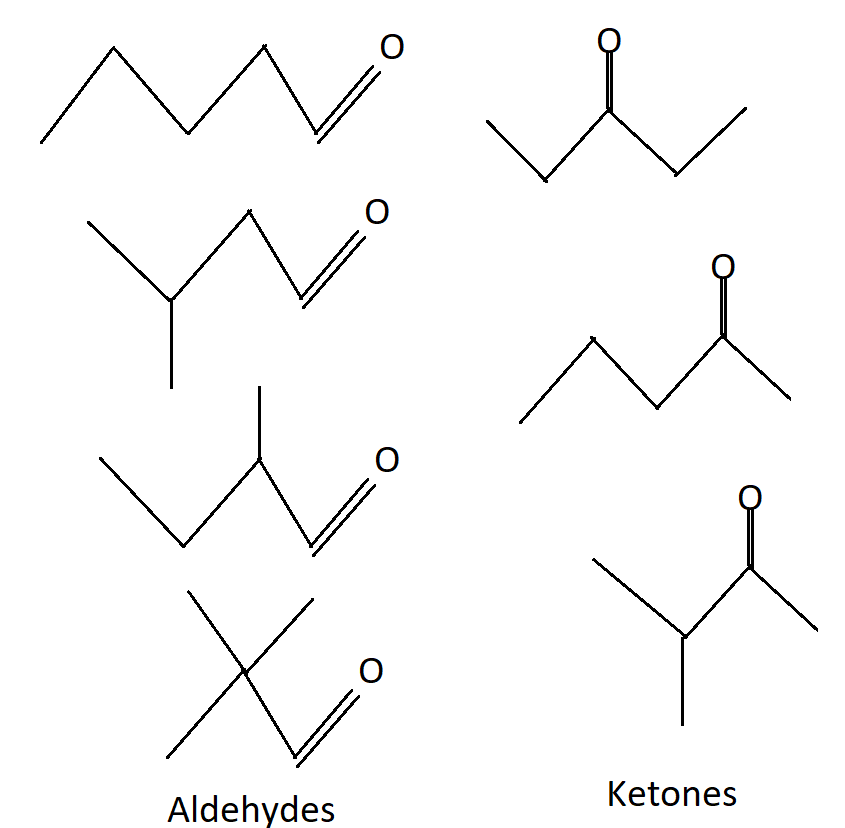
Draw The Structures Of 3 Ketones With Formula C5h10o C5h10o has a total of 7 isomers. four of them are aldehyde and three of them are ketone. the 4 aldehydes of c5h10o are: pentanal, 2 methylbutanal, 3 methylb. In this video, we have written the constitutional isomers of c5h10o that contains an aldehyde or ketone as a functional group in bond line notation including.

Draw The Structures Of 3 Ketones With Formula C5h10o Vrogue Co Question: draw and name all the isomers of c5h10o, aldehydes & ketones only. draw and name all the isomers of c5h10o, aldehydes & ketones only. here’s the best way to solve it. calculate the degree of unsaturation for the molecule c 5 h 10 o using the formula ( 2 c 2 − h 2) 1 to determine the number of double bonds or rings. #chemistry #chemistrymadeeasy #isomerismwriting possible isomers of aldehydes and ketones for the molecular formula c5.h10.ostructural formula and iupac nome. Question:. what are the possible aldehydes and ketones with molecular formula $\ce{c5h10o$ (including the stereoisomers)?. answer: 5 aldehydes and 3 ketones. the degree of unsaturation of the compound is 1, so the ketone part is understandable since you take a carbon chain of length 5 and we have 3 possibilities for attaching double bond oxygen. So c5h12 is the molecular formula for this compound. and this is another structural isomer. so it's a different molecule from the other two. so we have a total of three structural isomers that have the molecular formula c5h12. now let's draw all of the structural isomers that have the molecular formula c3h8o.

C5h10 Lewis Structure Isomers Question:. what are the possible aldehydes and ketones with molecular formula $\ce{c5h10o$ (including the stereoisomers)?. answer: 5 aldehydes and 3 ketones. the degree of unsaturation of the compound is 1, so the ketone part is understandable since you take a carbon chain of length 5 and we have 3 possibilities for attaching double bond oxygen. So c5h12 is the molecular formula for this compound. and this is another structural isomer. so it's a different molecule from the other two. so we have a total of three structural isomers that have the molecular formula c5h12. now let's draw all of the structural isomers that have the molecular formula c3h8o. Draw the structural formula of all the possible isomers of the compound with the molecular formula for c 3 h 6 o and also give their electron dot structures. view solution. q 5. The easiest way of determining if molecules are constitutional isomers is to quickly count the number of carbons and the degree of unsaturation (hydrogen deficiency index). if all the atoms are the same and molecules have the same hdi, then they are constitutional isomers. however, to be absolutely sure, especially for large molecules, we need.

Draw The Structures Of 3 Ketones With Formula C5h10o Draw the structural formula of all the possible isomers of the compound with the molecular formula for c 3 h 6 o and also give their electron dot structures. view solution. q 5. The easiest way of determining if molecules are constitutional isomers is to quickly count the number of carbons and the degree of unsaturation (hydrogen deficiency index). if all the atoms are the same and molecules have the same hdi, then they are constitutional isomers. however, to be absolutely sure, especially for large molecules, we need.

Comments are closed.