Copper Orbital Diagram Organicful
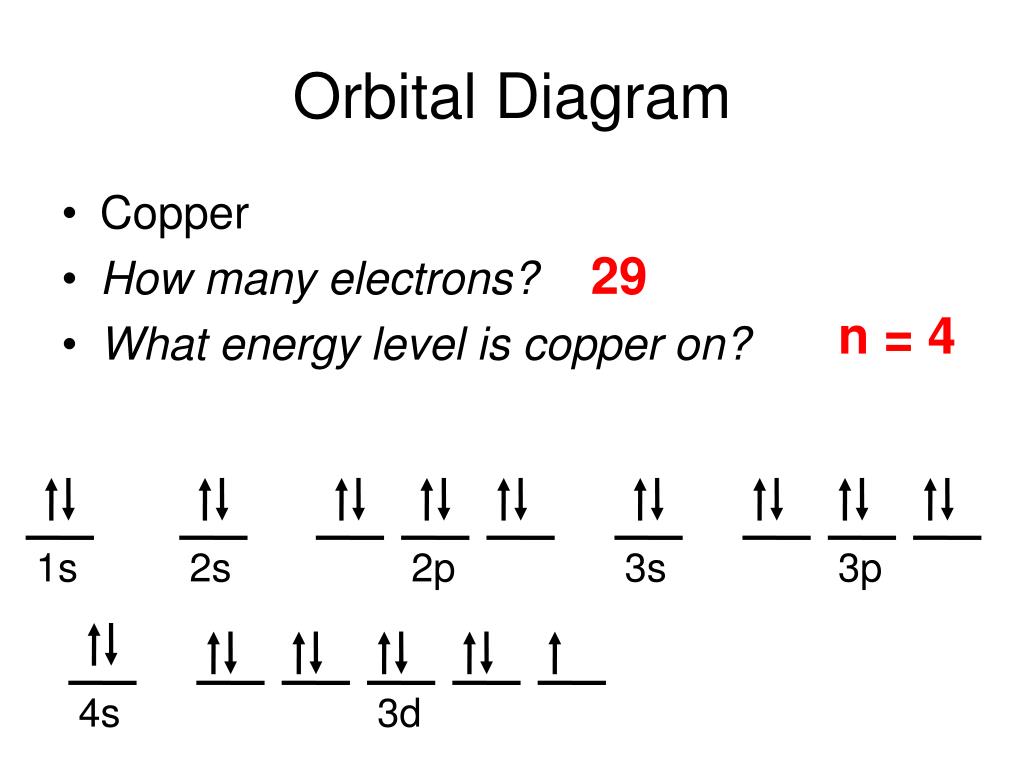
Copper Orbital Diagram Organicful Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for just . to do that we need to find the number of elect.
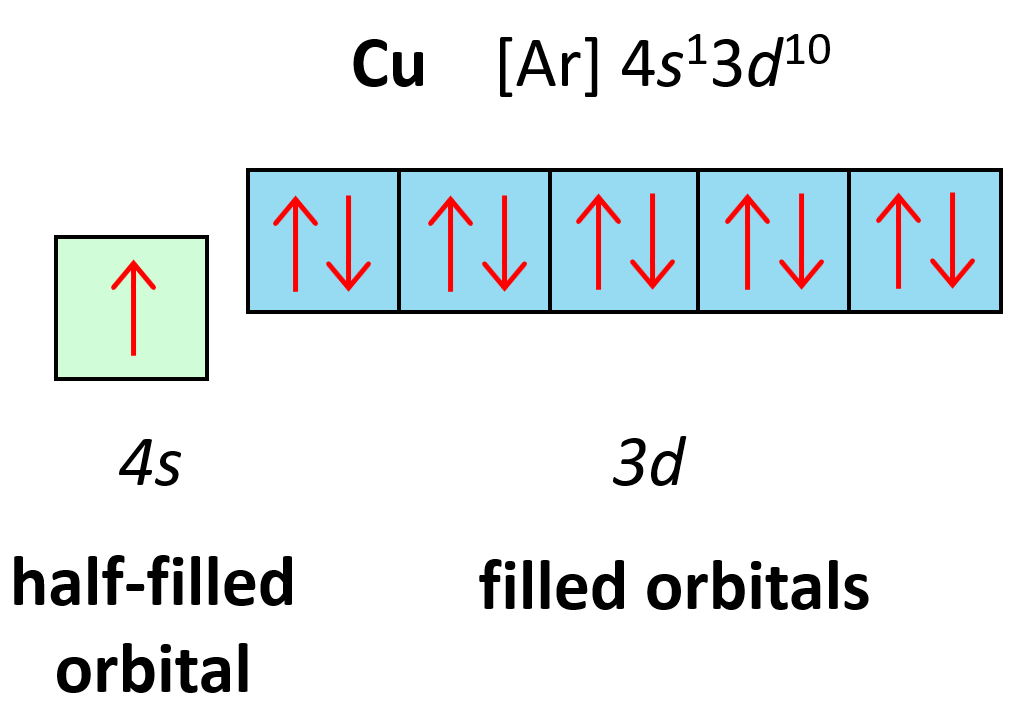
Electron Configuration Of Copper The copper atom donates an electron in the 4s orbital to form a copper ion(cu ). cu – e – → cu here, the electron configuration of copper ion(cu ) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. on the other hand, the copper atom donates an electron in the 4s orbital and an electron in the 3d orbital to convert copper ion(cu 2 ). 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the only difference is at the end of the configuration that is in the 3d and 4s shells. the atoms of copper have enough electrons which. The electron configuration for carbon is 1s22s22p2. an orbital box diagram can be written as well. boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. Orbital diagrams. an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. this is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.
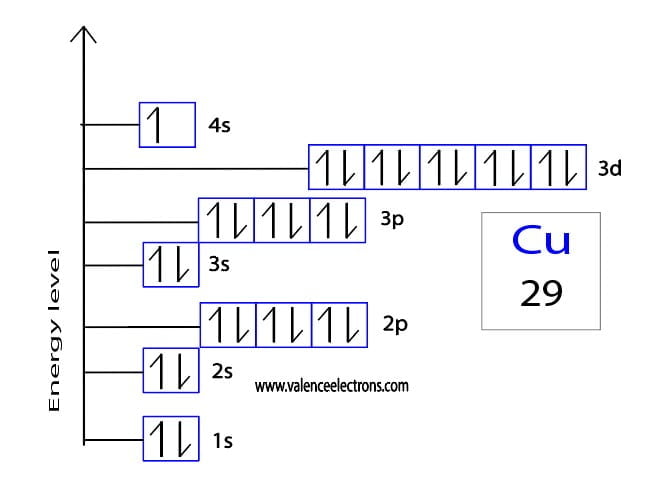
Orbital Diagram Of Copper The electron configuration for carbon is 1s22s22p2. an orbital box diagram can be written as well. boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. Orbital diagrams. an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. this is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. In the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p subshell encompasses six electrons, the 3s subshell contains two electrons, the 3p subshell carries six electrons, the 4s subshell holds one electron, and the 3d subshell carries ten electrons, totaling twenty nine electrons. The orbital diagram for copper (cu) can be represented as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d9. copper (cu) has an atomic number of 29, which means it has 29 electrons. in its ground state, copper has the electron configuration of [ar]4s 2 3d 9. this means that the 29 electrons are distributed among the various orbitals available in the copper.
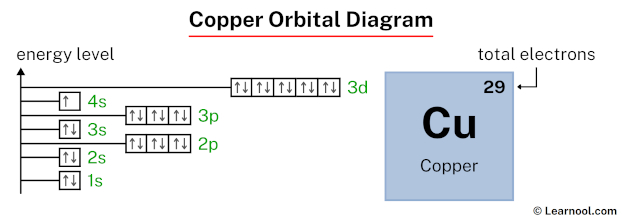
Copper Orbital Diagram Learnool In the copper orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p subshell encompasses six electrons, the 3s subshell contains two electrons, the 3p subshell carries six electrons, the 4s subshell holds one electron, and the 3d subshell carries ten electrons, totaling twenty nine electrons. The orbital diagram for copper (cu) can be represented as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d9. copper (cu) has an atomic number of 29, which means it has 29 electrons. in its ground state, copper has the electron configuration of [ar]4s 2 3d 9. this means that the 29 electrons are distributed among the various orbitals available in the copper.

Comments are closed.