Covalent Bond Definition Types Properties And Examples

Covalent Bond Definition Types And Examples Covalent bonds and ionic bonds are types of atomic bonds. these bonds are different in their properties and structure. covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation, while a bond between two ions is called an ionic bond. covalent vs ionic bonds. covalent bonding occurs between two non metallic atoms. Based on the number of shared electron pairs, there are three types of covalent bonds [1 6]: 1. single covalent bond. when one pair of electrons, or two electrons, are shared between the atoms, it is known as a single covalent bond or merely a single bond. examples: h 2, cl 2, br 2, i 2, hcl, nh 3, ch 4, and c 2 h 6. 2.

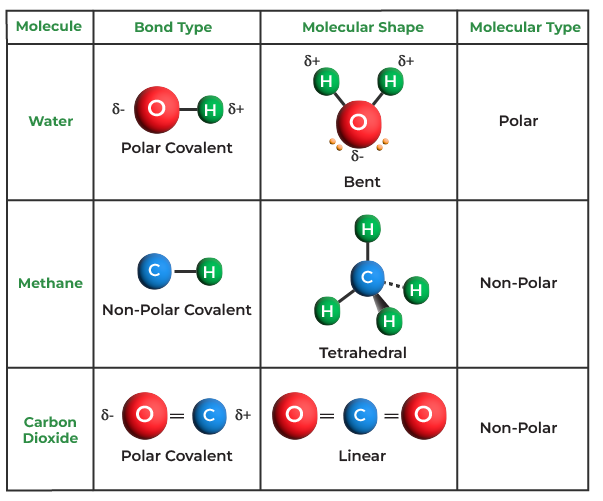
Covalent Bond Examples Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. the binding arises from the electrostatic attraction of their nuclei for the same electrons. a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. A covalent bond is a type of chemical bond characterized by two atoms sharing valence electrons. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms are on. An example of double covalent bonds is the bonding between carbon and oxygen atoms in co2. 3. triple covalent bond. a triple covalent bond is formed by the sharing of three pairs of electrons between the participating atoms. a triple covalent bond is indicated by (≡). A. depending on the number of shared electron pair or the bond occurs between two atoms, covalent bonds can be classified in 3 different types. single covalent bond. double covalent bond. triple covalent bond. b. depending on polarity covalent bond is classified in 2 different types: non polar covalent bond. polar covalent bond.
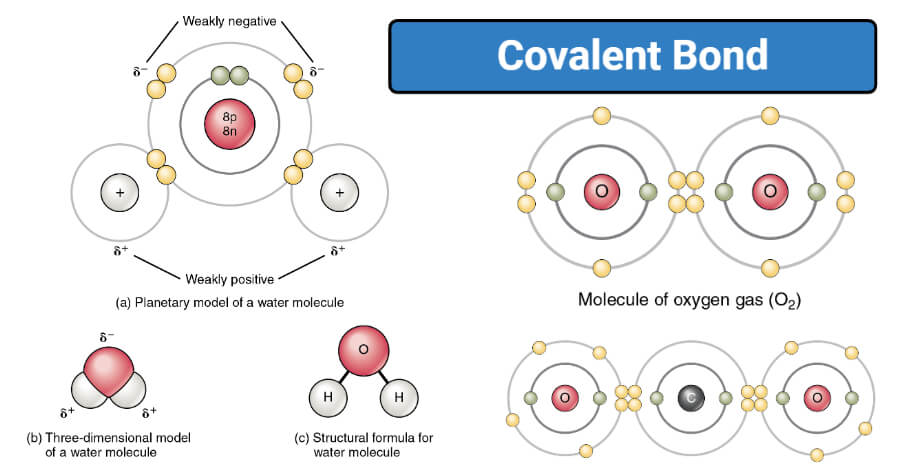
Covalent Bond Definition Properties Types Examples An example of double covalent bonds is the bonding between carbon and oxygen atoms in co2. 3. triple covalent bond. a triple covalent bond is formed by the sharing of three pairs of electrons between the participating atoms. a triple covalent bond is indicated by (≡). A. depending on the number of shared electron pair or the bond occurs between two atoms, covalent bonds can be classified in 3 different types. single covalent bond. double covalent bond. triple covalent bond. b. depending on polarity covalent bond is classified in 2 different types: non polar covalent bond. polar covalent bond. Some examples of covalent compounds – the construction of lewis structures of simple covalent compounds will be discussed. covalent bond in hydrogen, h 2 – a hydrogen molecule is made of two h atoms, each having one valence electron. – each contributes an electron to the shared pair and both atoms acquire stable helium configuration. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. these electron pairs are known as shared pairs or bonding pairs. the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. [ 1] for many molecules, the sharing of.

Covalent Bond Definition Properties Examples Facts Britannica Some examples of covalent compounds – the construction of lewis structures of simple covalent compounds will be discussed. covalent bond in hydrogen, h 2 – a hydrogen molecule is made of two h atoms, each having one valence electron. – each contributes an electron to the shared pair and both atoms acquire stable helium configuration. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. these electron pairs are known as shared pairs or bonding pairs. the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. [ 1] for many molecules, the sharing of.
Covalent Bond Definition Examples Types Properties Faqs

Comments are closed.