Covalent Bond Examples Science Struck

Covalent Bond Examples Science Struck Hydrogen molecule (h2) is a non polar covalent bond example, as an electron pair is equally shared between the two hydrogen atoms. ammonium chloride. ammonium chloride (nh4cl) is a coordinate covalent bond example, where both electrons required for bonding, are supplied by the same atom. here is a table listing molecules with polar and non. Examples of molecular compounds with their common names are; h 2 o – water, h 2 o 2 – hydrogen peroxide, ch 4 – methane, nh 3 – ammonia, and so on. in order to learn the naming convention, let’s take an example of the common molecular compound, water (h 2 o). for naming this binary compound, the name of the first element should be.
Covalent Bond Examples Science Struck Covalent bonds usually form between nonmetals. examples of covalent compounds include hydrogen (h 2 ), oxygen (o 2 ), carbon monoxide (co), ammonia (nh 3 ), water (h 2 o), and all organic compounds. there are compounds that contain both covalent and ionic bonds, such as potassium cyanide (kcn) and ammonium chloride (nh 4 cl). These are examples of covalent bonds and covalent compounds. covalent compounds also are known as molecular compounds. organic compounds, such as carbohydrates, lipids, proteins, and nucleic acids, are all examples of molecular compounds. you can recognize these compounds because they consist of nonmetals bonded to each other. If the electronegativity difference is 2 or more, the elements form ionic bonds. examples of covalent compounds include: o 2 – oxygen. cl 2 – chlorine. pcl 3 – phosphorus trichloride. ch 3 ch 2 oh – ethanol. o 3 – ozone. h 2 – hydrogen. h 2 o – water. The atoms that are strongly bonded (ionic or covalent bonds) are said to have low potential chemical energy as compared to those with weaker bonds. this is because, for stronger bonds to break, a greater amount of external energy is required. whereas in the case of weaker bonds (like van der waal type), less energy is required for breaking.
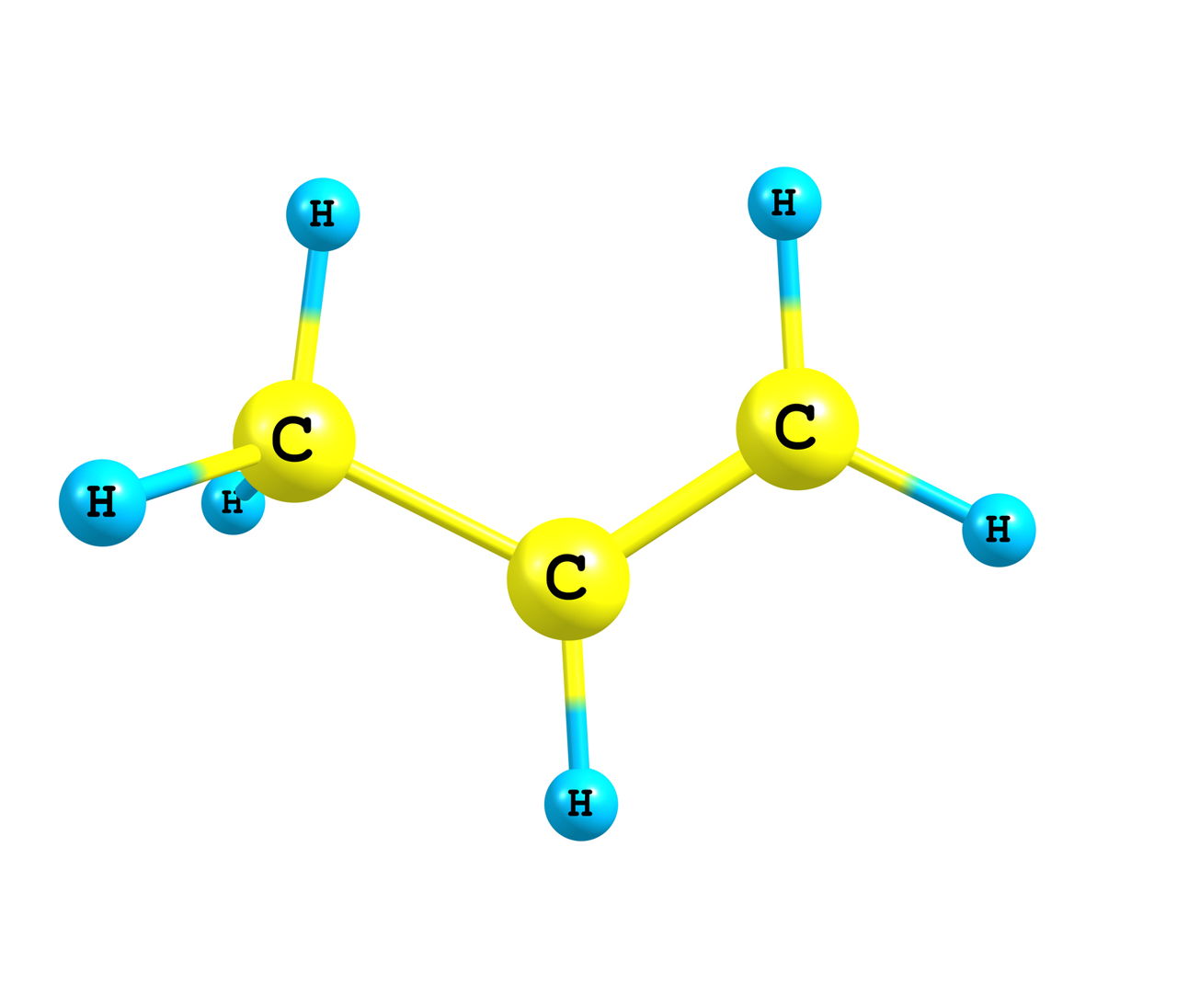
Covalent Bond Examples Science Struck If the electronegativity difference is 2 or more, the elements form ionic bonds. examples of covalent compounds include: o 2 – oxygen. cl 2 – chlorine. pcl 3 – phosphorus trichloride. ch 3 ch 2 oh – ethanol. o 3 – ozone. h 2 – hydrogen. h 2 o – water. The atoms that are strongly bonded (ionic or covalent bonds) are said to have low potential chemical energy as compared to those with weaker bonds. this is because, for stronger bonds to break, a greater amount of external energy is required. whereas in the case of weaker bonds (like van der waal type), less energy is required for breaking. A covalent bond is formed between two atoms by sharing electrons. the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. hydrogen is an exception to the octet rule. h forms only one bond because it needs only two electrons. Based on the number of shared electron pairs, there are three types of covalent bonds [1 6]: 1. single covalent bond. when one pair of electrons, or two electrons, are shared between the atoms, it is known as a single covalent bond or merely a single bond. examples: h 2, cl 2, br 2, i 2, hcl, nh 3, ch 4, and c 2 h 6. 2.

Covalent Bond Examples Science Struck A covalent bond is formed between two atoms by sharing electrons. the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. hydrogen is an exception to the octet rule. h forms only one bond because it needs only two electrons. Based on the number of shared electron pairs, there are three types of covalent bonds [1 6]: 1. single covalent bond. when one pair of electrons, or two electrons, are shared between the atoms, it is known as a single covalent bond or merely a single bond. examples: h 2, cl 2, br 2, i 2, hcl, nh 3, ch 4, and c 2 h 6. 2.

Covalent Bond Examples Science Struck

Comments are closed.