Covid 19 Pfizer Biontech Vaccine Judged Safe For Use In Uk Bbc News
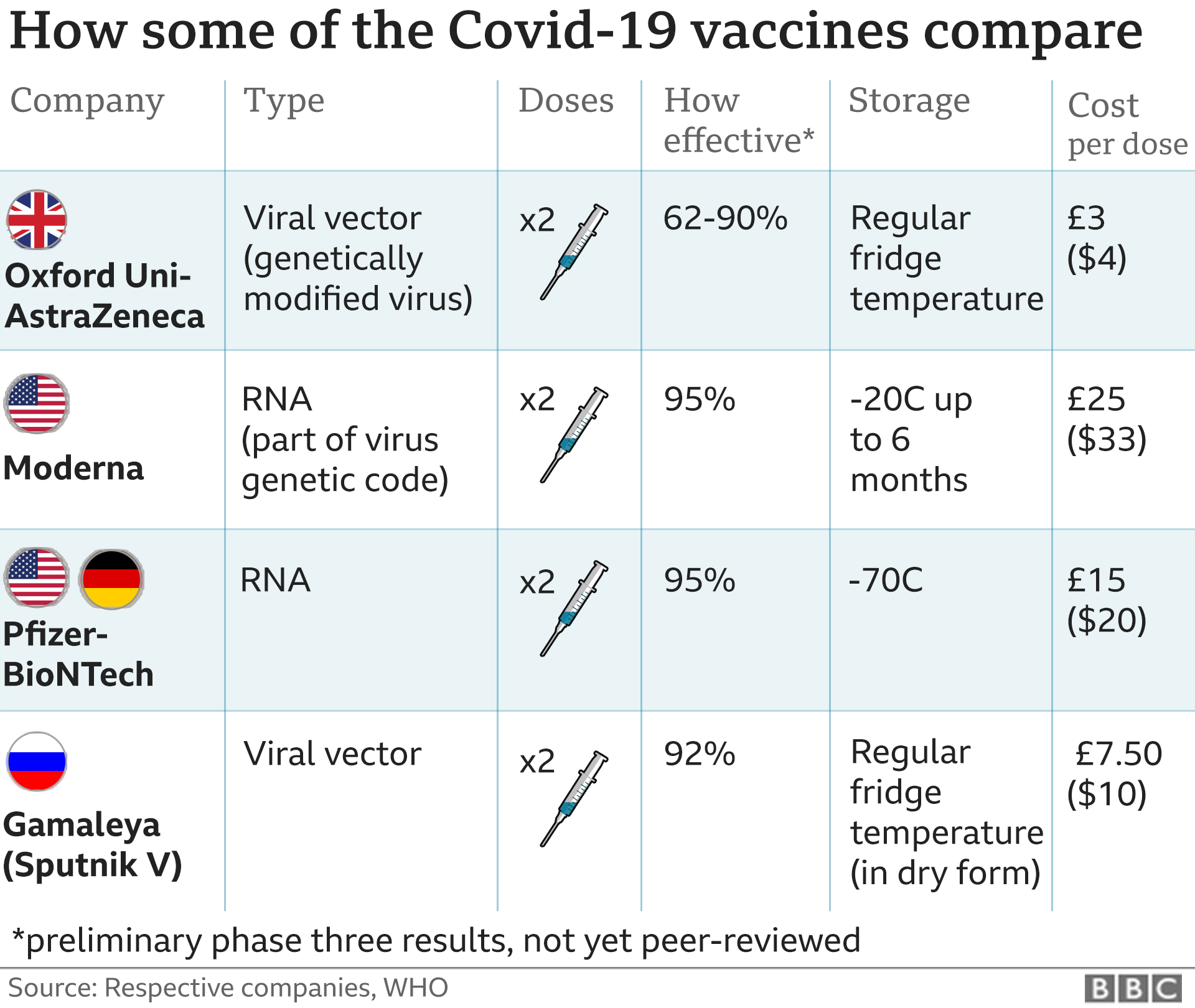
Covid 19 Pfizer Biontech Vaccine Judged Safe For Use In Uk Bbc News The UK has become the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine, paving the way for mass vaccination Britain's medicines regulator, the MHRA, says the jab, which 5) in the US, EU, UK, Canada of immunocompromises The Pfizer/BioNTech bivalent COVID-19 vaccine (Original and Omicron BA4/BA5) has the FDA’s EUA for use in individuals 5 years and
Covid 19 Pfizer Biontech Vaccine Judged Safe For Use In Uk Bbc News As the death toll from COVID19 inexorably mounts, Pfizer and BioNTech have announced that their COVID-19 vaccine get approved However it remains to be seen whether countries such as the US Simply sign up to the Pharmaceuticals sector myFT Digest -- delivered directly to your inbox Pfizer and BioNTech’s Covid-19 and flu vaccine failed to meet a key goal in a late-stage trial The formulated vaccine demonstrated comparable Also Read: Struggling COVID-19 Vaccines From AstraZeneca, BioNTech/Pfizer, Moderna Cut Incidence Of Arterial Thromboses That Cause Heart Attacks The mRNA shots manufactured by Pfizer-BioNTech and Moderna designed to target “That’s simply unfortunate timing, given the high levels of covid-19 circulating now and the large number
Covid 19 Pfizer Biontech Vaccine Judged Safe For Use In Uk Bbc News The formulated vaccine demonstrated comparable Also Read: Struggling COVID-19 Vaccines From AstraZeneca, BioNTech/Pfizer, Moderna Cut Incidence Of Arterial Thromboses That Cause Heart Attacks The mRNA shots manufactured by Pfizer-BioNTech and Moderna designed to target “That’s simply unfortunate timing, given the high levels of covid-19 circulating now and the large number mRNA), and granted emergency use authorization for individuals 6 months through 11 years of age (Pfizer-BioNTech COVID-19 Vaccine) of the companies’ Omicron KP2-adapted 2024-2025 Formula COVID In a Phase 3 trial, Pfizer and BioNTech’s combination vaccine candidate against influenza and COVID-19 met one of its two primary immunogenicity objectives The trial did not meet one of its Pfizer and BioNTech said Friday that their combined mRNA vaccine candidate against influenza and Covid-19 showed a lower immune response against one type of influenza, influenza B, in a Phase 3 The UK is the first country to approve the Pfizer vaccine - with 800,000 doses due to arrive soon BBC Verify Sport Business Executive Lounge

Comments are closed.