Covid 19 Vaccine And Antiviral Drug Development Fpm
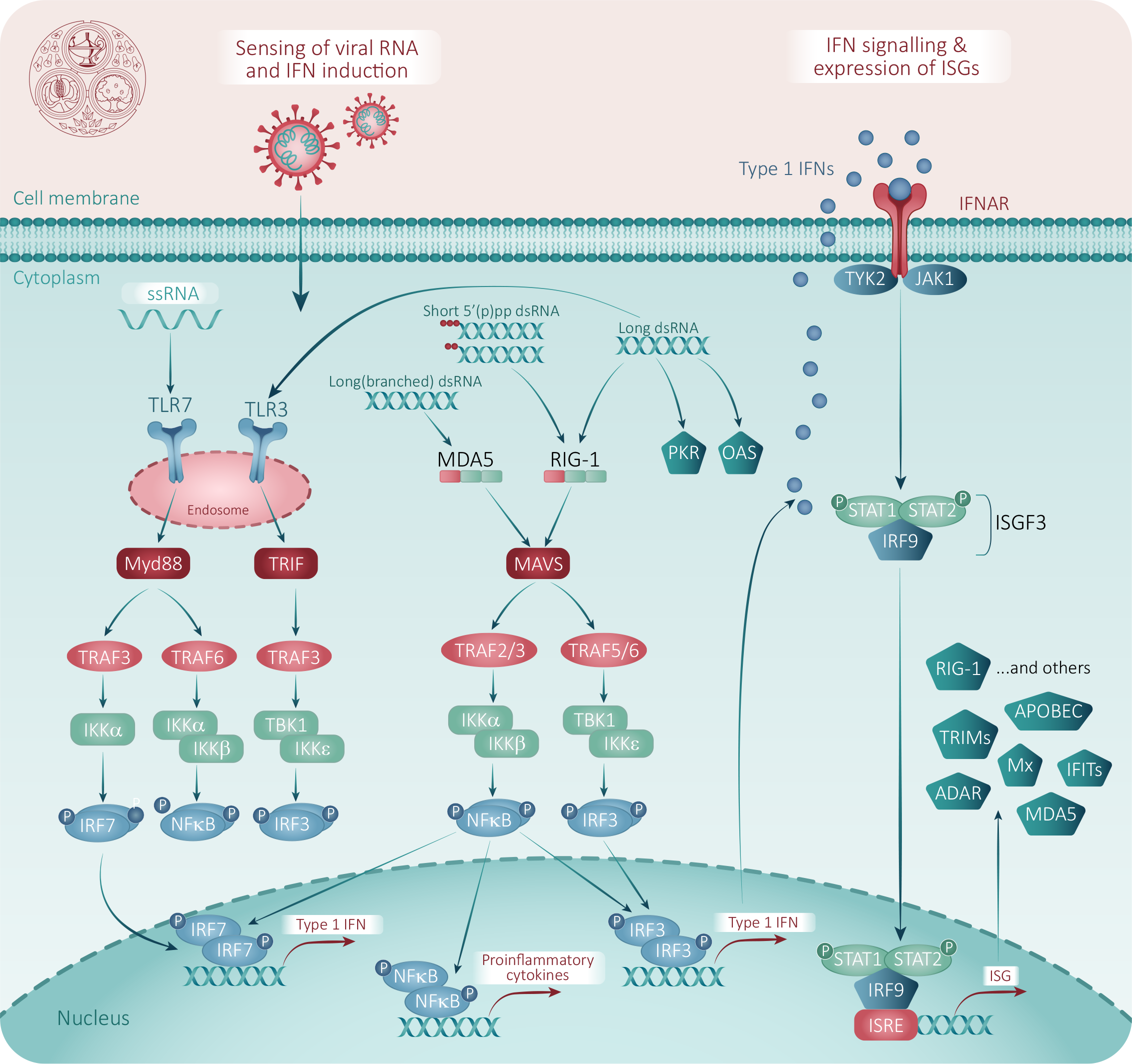
Covid 19 Vaccine And Antiviral Drug Development Fpm Lopinavir ritonavir. lopinavir ritonavir, a protease inhibitor used to treat hiv aids, was found to block covid 19 infection in vitro at low micromolar concentration, with a half maximal effective concentration (ec50) of 8.5 μm. in contrast in vitro inhibitory concentrations vs hiv are in the low nm range (~6nm). This article addresses the current debates over covid 19 antivirals and vaccine boosters and takes other considerations into account: whether the widespread use of antivirals will be possible and advisable. what might happen if long term efficacy of the vaccine is shown to be low, and how do we develop therapeutics to address the unmet need.

Covid 19 Vaccine And Antiviral Drug Development Fpm So far, a few small molecule antiviral drugs (nirmatrelvir–ritonavir, remdesivir and molnupiravir) and 11 monoclonal antibodies have been marketed for the treatment of covid 19, mostly requiring. Before the covid 19 pandemic, the focus of antiviral development was on human immunodeficiency virus (hiv) and hepatitis c virus (hcv), accounting for more than 67% of approved antivirals 5. the. Vaccine development is a complex endeavour. it involves multiple phases, including an initial design stage, preclinical studies, phases 1–3 of clinical trial testing, approval for human use, and. Developing covid 19 vaccines at pandemic speed lurie n et al. nejm doi: 10.1056 march 30, 2020. global coalition to accelerate covid 19 clinical research in resource limited settings. the lancet. published online april 2 2020. the covid 19 clinical research coalition.
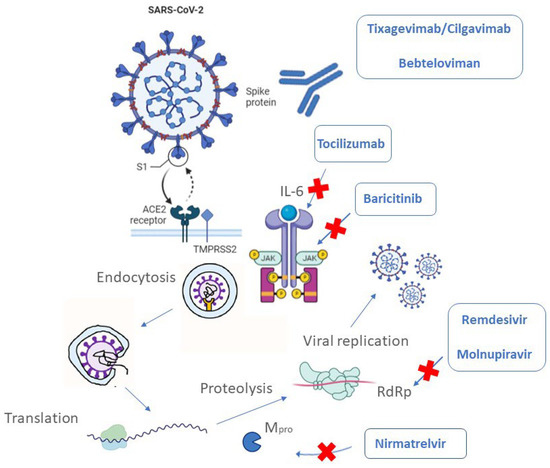
Biomedicines Free Full Text Overview Of Antiviral Drug Therapy For Vaccine development is a complex endeavour. it involves multiple phases, including an initial design stage, preclinical studies, phases 1–3 of clinical trial testing, approval for human use, and. Developing covid 19 vaccines at pandemic speed lurie n et al. nejm doi: 10.1056 march 30, 2020. global coalition to accelerate covid 19 clinical research in resource limited settings. the lancet. published online april 2 2020. the covid 19 clinical research coalition. The drug discovery strategy bears the brunt of the covid 19 outbreak is the test of existing broad spectrum antiviral agents that have been harnessed to cure other viral infections via utilizing standard tests for measuring the effects of drugs on the cytopathy, virus production along with plaque generation in live and pseudotyped covs . Keywords: covid 19, sars cov 2, therapeutics, mrna vaccines, small molecule antiviral drugs, neutralizing antibodies, vaccine development introduction the first coronavirus disease 2019 (covid 19) infections were reported in late december 2019, and the disease spread rapidly around the world, echoing the fearsome global outbreak of “spanish.

Comments are closed.