Cu Ni Example
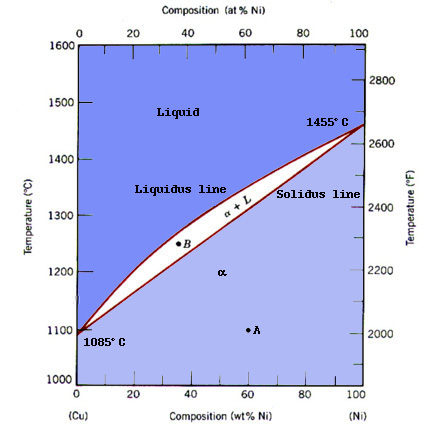
Cu Ni Example The most ancient cu ni coin that has been saved for posterity comes from the period around bc 235. it was found in bactria and consists of an alloy similar e.g. to the former german 50 pfennig and 1 dm pieces (approximately 75% cu and 25% ni). these and many other old coins are outstanding examples for the high corrosion resistance of cu ni alloys. The cu ni binary system is a well known example, which consists of copper (cu) and nickel (ni) as its primary components. this system exhibits several key phases that are of great interest in various industries and research fields.
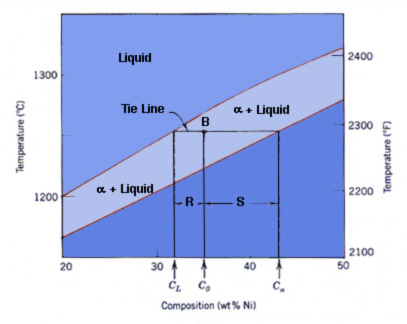
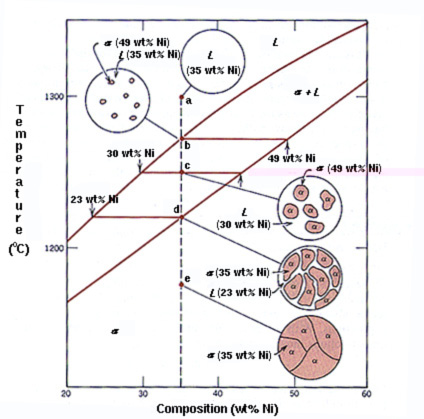
Cu Ni Example The composition of the liquid phase, c l, is 32 wt% ni 68 wt% cu. thusly, the alpha phase, c alpha, has a composition of 43 wt% ni 57 wt% cu. through the use of the lever rule, one can also determine the amount of each phase present. the lever rule is an expression which allows one to compute the phase amounts in a two phase alloy. Non marine applications include coinage such as silver coins and brake tubing. for example: 1 euro coin, 2 euro coin, and 5 us cent. the 1 euro bimetallic coin’s external part is made of nickel brass alloy (75% copper, 20% zinc, 5% nickel) and the internal part is made of cupronickel alloy (75% copper, 25% nickel ). Nickel copper (ni cu) alloys exhibit simultaneously high strength and toughness, excellent corrosion resistance, and may show good wear resistance. therefore, they are widely used in the chemical, oil, and marine industries for manufacturing of various components of equipment, such as: drill collars, pumps, valves, impellers, fixtures, pipes, and, particularly, propeller shafts of marine vessels. Each point on the graph represents a unique combination of temperature and pressure where an equilibrium exists between two phases. for example, when p = 0 bar and t = 900°c (the solidus line), all of the cu ni alloy system consists only of its liquid form. on the other hand, when p = 0 bar and t = 500°c (the liquidus line), all the systems.
Cu Ni Example Nickel copper (ni cu) alloys exhibit simultaneously high strength and toughness, excellent corrosion resistance, and may show good wear resistance. therefore, they are widely used in the chemical, oil, and marine industries for manufacturing of various components of equipment, such as: drill collars, pumps, valves, impellers, fixtures, pipes, and, particularly, propeller shafts of marine vessels. Each point on the graph represents a unique combination of temperature and pressure where an equilibrium exists between two phases. for example, when p = 0 bar and t = 900°c (the solidus line), all of the cu ni alloy system consists only of its liquid form. on the other hand, when p = 0 bar and t = 500°c (the liquidus line), all the systems. Copper nickel (also known as cupronickel) alloys are widely used for marine applications due to their excellent resistance to seawater corrosion, low macrofouling rates, and good fabricability. they have provided reliable service for decades while offering effective solutions to today’s technological challenges. the addition of nickel to. Cu–ni–sn alloys have been widely used in the aerospace industry, the electronics industry, and other fields due to their excellent electrical and thermal conductivity, high strength, corrosion and wear resistance, etc., which make cu–15ni–8sn alloys the perfect alternative to cu–be alloys. this paper begins with how cu–ni–sn alloys are prepared. then, the microstructural features.

The Phase Diagram Of Cuвђ Ni System Download Scientific Diagram Copper nickel (also known as cupronickel) alloys are widely used for marine applications due to their excellent resistance to seawater corrosion, low macrofouling rates, and good fabricability. they have provided reliable service for decades while offering effective solutions to today’s technological challenges. the addition of nickel to. Cu–ni–sn alloys have been widely used in the aerospace industry, the electronics industry, and other fields due to their excellent electrical and thermal conductivity, high strength, corrosion and wear resistance, etc., which make cu–15ni–8sn alloys the perfect alternative to cu–be alloys. this paper begins with how cu–ni–sn alloys are prepared. then, the microstructural features.

Comments are closed.