Cu Oac 2 Catalyzed Coupling Of Aromatic Cвђ H Bonds

Cu Oac 2 Catalyzed Coupling Of Aromatic Cвђ H Bonds Abstract. cu catalyzed coupling of aryl c–h bonds with arylboron reagents was accomplished using a readily removable directing group, which provides a useful method for the synthesis of biaryl compounds. the distinct transmetalation step in this cu catalyzed c–h coupling with aryl borons provides unique evidence for the formation of an aryl. Cu(oac)2 catalyzed coupling of aromatic c h bonds with arylboron reagents. sign in | create an account. orcid.org. europe pmc. menu. about. about europe pmc.
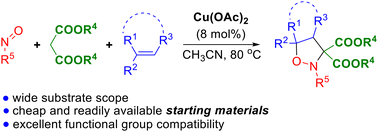
Cu Oac 2 Catalyzed Three Component Cycloaddition Of Malonates Hirano miura reported cu(oac) 2 catalyzed intramolecular aromatic c–h amination of biaryl 2 picolinamide for synthesis of carbazoles with mno 2 as the stoichiometric terminal oxidant to realize the catalytic turnover . 78 the reaction is initiated by aromatic c–h cupration by copper(ii)–picolinamide chelate complex 58 to afford organo cu(ii) intermediate 59. In 2007, fu and co workers [42] reported the copper catalyzed, tert butyl hydroperoxide (tbhp) assisted c–h amidation of tertiary amines 1. by heating at 80 °c, the c–h bond in dimethylaniline underwent direct amidation to provide products 3 in the presence of amides 2. on the other hand, the dephenylation transformation via c–c bond. A base and additive free method to access n arylated product by reacting substituted aryl boronic acids 32 with amines 2 or imides 70 employing inexpensive cu(oac) 2 ·h 2 o [bmim][bf 4] was reported by the group of kantam and co workers, scheme 31 [87]. this simple and recyclable protocol for the cross coupling was further extended to imides, amines, amides, and sulfonamides to broaden the. 2 insertion of c≡c and c=c bonds into [cp*m (c^x)] the rh (iii) and ir (iii) catalyzed couplings with olefins and alkynes are, by far, the most documented transformations. insertion of such unsaturated coupling partners into the c–m bond of metallacyclic species was firstly extensively studied [ 23 – 25 ].

Cu Oac 2 Catalyzed Aerobic Oxidative Dehydrogenation 55 Off A base and additive free method to access n arylated product by reacting substituted aryl boronic acids 32 with amines 2 or imides 70 employing inexpensive cu(oac) 2 ·h 2 o [bmim][bf 4] was reported by the group of kantam and co workers, scheme 31 [87]. this simple and recyclable protocol for the cross coupling was further extended to imides, amines, amides, and sulfonamides to broaden the. 2 insertion of c≡c and c=c bonds into [cp*m (c^x)] the rh (iii) and ir (iii) catalyzed couplings with olefins and alkynes are, by far, the most documented transformations. insertion of such unsaturated coupling partners into the c–m bond of metallacyclic species was firstly extensively studied [ 23 – 25 ]. The direct oxygenation of a structurally well defined aryl cu(iii) complex 27a with various carboxylates at room temperature was reported by wang in 2009. 16b in 2009, ohno and co workers reported a cu(oac) 2 mediated ortho functionalization of c(sp 2)–h bonds using tetrahydropyrimidine as the directing group, where the ortho hydroxylation of aromatic c–h bonds followed by cyclization with. The reaction with benzamide 2h using a catalytic amount of cu(oac) 2 formed product 3h; however, the starting material 1 was not effectively consumed. this may be due to the binding of cu(ii) to the carbonyl oxygen and imino nitrogen in the product. this problem was circumvented by using 2 equiv of cu(oac) 2 (entry 8). both electron withdrawing.

Comments are closed.