Cu Oac 2 Or Cutc Catalyzed Intermolecular 1 4 Difunctionalization Of

Cu Oac 2 Or Cutc Catalyzed Intermolecular 1 4 Difunctionalization Of Download scientific diagram | cu(oac)2 or cutc catalyzed intermolecular 1,4 difunctionalization of 1,3 enynes from publication: copper mediated chemo‐ and stereoselective cyanation reactions. A the mixture of 1 (0.2 mmol), arsih 3 (0.4 mmol), cu(oac) 2 thf meoh (1 1), n 2, 25 min. iii) cutc (10 mol %), cy m. cu catalyzed reductive gem difunctionalization of terminal alkynes via.
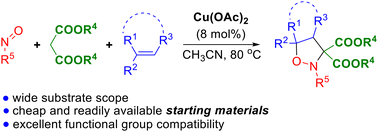
Cu Oac 2 Catalyzed Three Component Cycloaddition Of Malonates The reverse sequence, that is, carbocupration triggered difunctionalization of alkynes, was reported by li’s group in 2011 (scheme 29). 38 they developed cucl 2 catalyzed oxidative cyclization of 1,6 enyn 3 ones 105. 1,4 addition of h 2 o to the enone moiety generated copper enolate 108, which attacked the c c triple bond in an intramolecular. Notably, no desired 1,3 diene 5 was detected when we used cs 2 co 3 32,33 or k 3 po 4 36,37 as the base (table 2, entries 8 9), which could effectively promote nickel catalyzed or palladium. The observed regioselectivity towards 1,4 difunctionalization is due to the steric repulsions between the introduced aryl group and the spiro scaffold in 1,2 difunctionalization. 1. introduction alkenes are ubiquitous in all facets of chemistry and thus are privileged building blocks in organic synthesis. in recent years, radical reactions are experiencing a renaissance due to their high reactivity, chemoselectivity and functional group tolerance. 1 thus the radical involved 1,2 difunctionalization of alkenes, simultaneously installing two functional groups at vicinal.

Copper Amine Complex Solution As A Low Emission Catalyst For Flexible The observed regioselectivity towards 1,4 difunctionalization is due to the steric repulsions between the introduced aryl group and the spiro scaffold in 1,2 difunctionalization. 1. introduction alkenes are ubiquitous in all facets of chemistry and thus are privileged building blocks in organic synthesis. in recent years, radical reactions are experiencing a renaissance due to their high reactivity, chemoselectivity and functional group tolerance. 1 thus the radical involved 1,2 difunctionalization of alkenes, simultaneously installing two functional groups at vicinal. The radical involved 1,2 difunctionalization of alkenes has developed into a robust tool for preparation of complex organic molecules. despite significant advances in this area, the catalytic asymmetric version still remains a challenging task mainly due to the difficulty in the stereocontrol of the highly r. A copper catalyzed remote benzylic c–h functionalization strategy enabling 1,2 difunctionalization of alkenes with 2 methylbenzeneamides and nucleophiles, including alcohols, indoles, pyrroles, and the intrinsic amino groups, is reported, which is characterized by its redox neutral conditions, exquisite site selectivity, broad substrate scope, and wide utilizations of late stage modifying.

Organosulfide Catalyzed Enantioselective Intermolecular Iodinative The radical involved 1,2 difunctionalization of alkenes has developed into a robust tool for preparation of complex organic molecules. despite significant advances in this area, the catalytic asymmetric version still remains a challenging task mainly due to the difficulty in the stereocontrol of the highly r. A copper catalyzed remote benzylic c–h functionalization strategy enabling 1,2 difunctionalization of alkenes with 2 methylbenzeneamides and nucleophiles, including alcohols, indoles, pyrroles, and the intrinsic amino groups, is reported, which is characterized by its redox neutral conditions, exquisite site selectivity, broad substrate scope, and wide utilizations of late stage modifying.

Cu Catalyzed Regioselective 1 4 Difunctionalization Of 1 3 Enyne

Comments are closed.