Dccr Phase 3 Clinical Trial Overview
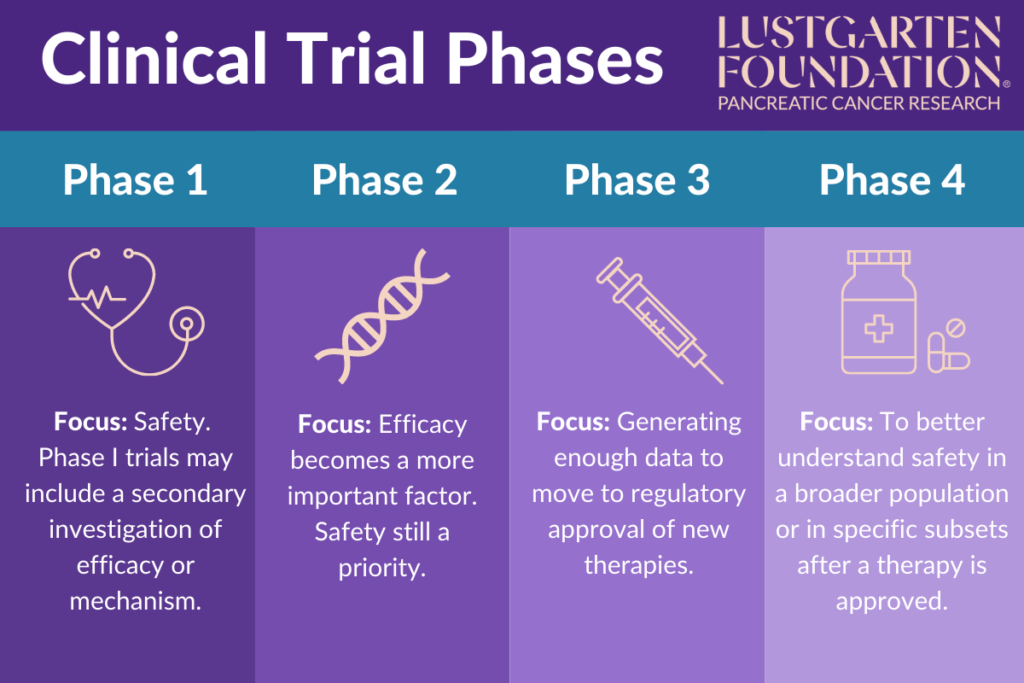
Clinical Trial Phases The phase 3 destiny pws trial, conducted at sites in the u.s. and the u.k., evaluated the effects of daily dccr tablets against a placebo among 127 pws patients, 4 years and older, over about three months. after completing the trial, 115 participants enrolled in the c602 study, where all continued treatment with dccr for up to four years. The safety profile of dccr in c601 was generally consistent with the known profile of diazoxide and prior experience with dccr. treatment emergent adverse events (teaes) were reported in 70 (83.3%) dccr subjects and 31 (73.8%) placebo subjects. teaes that were reported more frequently in the dccr group vs. placebo and occurred in at least 5% of.

March 2022 Biostatistics Bioinformatics Services Soleno therapeutics is conducting a phase 3 study of dccr to measure the efficacy of the dccr for treating hyperphagia in pws. the study seeks to enroll 105 patients with pws ages 4 years and older. the study will be a 15 week, randomized, double blind, placebo controlled study of diazoxide choline controlled release tablet (dccr) and will. The dccr development program is supported by data from five completed phase i clinical studies in healthy volunteers and three completed phase ii clinical studies, one of which was in pws patients. in the pws phase ii study, dccr showed promise in addressing hyperphagia, the hallmark symptom of pws, as well as several other symptoms such as. The dccr development program is supported by data from five completed phase 1 clinical studies in healthy volunteers and three completed phase 2 clinical studies, one of which was in pws patients. in the pws phase 3 study, dccr showed promise in addressing hyperphagia, the hallmark symptom of pws, as well as several other symptoms such as. In this hour long video, the ceo of soleno therapeutics and researcher dr. jennifer miller go into detail on the design and results of the dccr phase 3 clinical trial. the session also covers next steps for making the drug available to treat pws, discussed by larry bauer, who worked in the rare diseases program at the fda.

Dccr For Royer Syndrome Clinical Trial 2022 Power The dccr development program is supported by data from five completed phase 1 clinical studies in healthy volunteers and three completed phase 2 clinical studies, one of which was in pws patients. in the pws phase 3 study, dccr showed promise in addressing hyperphagia, the hallmark symptom of pws, as well as several other symptoms such as. In this hour long video, the ceo of soleno therapeutics and researcher dr. jennifer miller go into detail on the design and results of the dccr phase 3 clinical trial. the session also covers next steps for making the drug available to treat pws, discussed by larry bauer, who worked in the rare diseases program at the fda. Data from dccr treated participants in the c602 c602 cohort were compared to results from a cohort of comparable participants from path using the same caregiver completed questionnaires to measure hyperphagia (hyperphagia questionnaire for clinical trials [hq ct]) and pws related behaviors (the prader willi syndrome profile [pwsp]) in six. Soleno therapeutics has announced top line results from the company’s phase 3 trial, destiny pws (c601), evaluating once daily diazoxide choline controlled release (dccr) tablets for patients with prader willi syndrome (pws). the results of the study are mixed, with some potential bright spots, but not a clear cut "win.".

All About Cancer Clinical Trials Trial Safety Measures Masonic Data from dccr treated participants in the c602 c602 cohort were compared to results from a cohort of comparable participants from path using the same caregiver completed questionnaires to measure hyperphagia (hyperphagia questionnaire for clinical trials [hq ct]) and pws related behaviors (the prader willi syndrome profile [pwsp]) in six. Soleno therapeutics has announced top line results from the company’s phase 3 trial, destiny pws (c601), evaluating once daily diazoxide choline controlled release (dccr) tablets for patients with prader willi syndrome (pws). the results of the study are mixed, with some potential bright spots, but not a clear cut "win.".

Phases Of Clinical Trial Summary

Comments are closed.