Determination Of Concentration Of Kmno4 Solution Meity Olabs

Determination Of Concentration Of Kmno4 Solution Meity Olabs Youtube This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.edu.in ?sub=73&brch=8&si. This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.co.in ?sub=73&brch=8&sim.

Determination Of Concentration Of Kmno4 Soution Using Ferrous Ammonium To determine the strength of kmno4 solution by titrating it against a standard solution of: 1.oxalic acid 2.ferrous ammonium sulphate (mohr’s salt) హోమ్ గురించి. A solution whose concentration is known, is called a standard solution. the substance used to prepare a standard solution is called the primary standard. oxalic acid and sodium carbonate are some examples. 2. concentration of a solution. concentration of a solution is defined as the amount of a solute present in a definite volume of the solvent. Experiment on determination of chemical oxygen demand … 12 0 experiment on determination of chemical oxygen demand absolute zero determination of concentration of kmno4 solution using oxalic acid meity olabs titration experiment \u0026 calculate the molarity of acetic acid in vinegar titration: practical and 12 0 experiment on. 2. concentration of a solution concentration of a solution is defined as the amount of a solute present in a definite volume of the solvent. concentration of a solution can be expressed in different ways. normality: normality of a solution is defined as the number of gram equivalent of solute per litre of the solution. it is denoted by ‘n’.

Determination Of Concentration Of Kmno4 Solution Using Oxalic Acid Experiment on determination of chemical oxygen demand … 12 0 experiment on determination of chemical oxygen demand absolute zero determination of concentration of kmno4 solution using oxalic acid meity olabs titration experiment \u0026 calculate the molarity of acetic acid in vinegar titration: practical and 12 0 experiment on. 2. concentration of a solution concentration of a solution is defined as the amount of a solute present in a definite volume of the solvent. concentration of a solution can be expressed in different ways. normality: normality of a solution is defined as the number of gram equivalent of solute per litre of the solution. it is denoted by ‘n’. V 2 and v 1 are the volume of potassium permanganate and oxalic acid solutions used in the titration. therefore, kmno 4 = oxalic acid. 5m 2 v 2 = 2m 1 v 1. m 2 = (2m 1 v 1 5m 2 v 2) the strength of kmno 4 is calculated by using the molarity. strength = molarity x molar mass. 1. prepare a standard 0.1m aqueous kmno4 solution. this is used as stock solution. 2. from the above stock solution, prepare five different concentrations of kmno4 solutions (say, 0.0001m, 0.00025m, 0.0005m, 0.00075m, and 0.001m). switch on the computer and the instrument powers; wait for 30 minutes for 'warm up' of the instrument. 3.
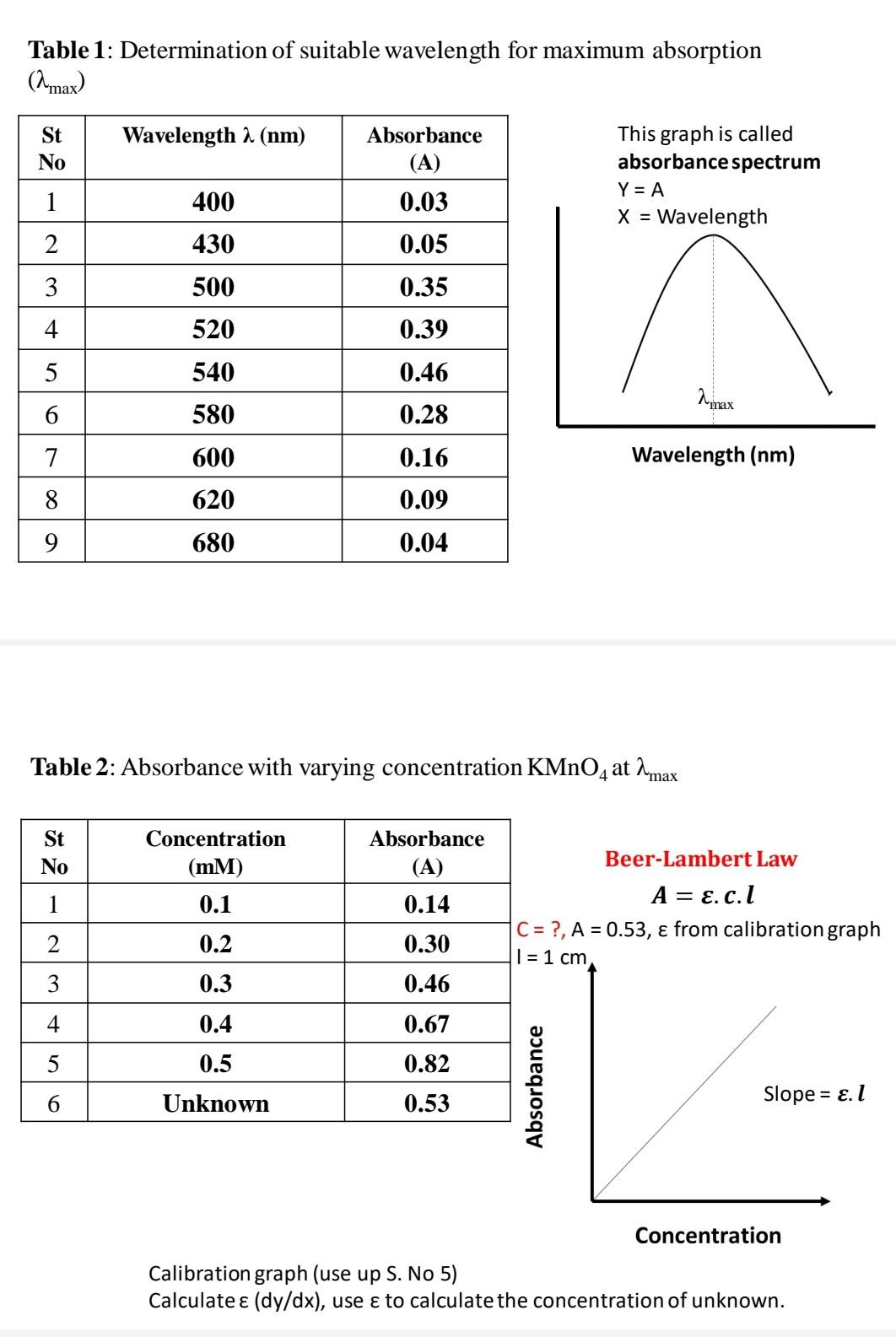
Solved Concentration Determination Of Kmno4 Solution By Chegg V 2 and v 1 are the volume of potassium permanganate and oxalic acid solutions used in the titration. therefore, kmno 4 = oxalic acid. 5m 2 v 2 = 2m 1 v 1. m 2 = (2m 1 v 1 5m 2 v 2) the strength of kmno 4 is calculated by using the molarity. strength = molarity x molar mass. 1. prepare a standard 0.1m aqueous kmno4 solution. this is used as stock solution. 2. from the above stock solution, prepare five different concentrations of kmno4 solutions (say, 0.0001m, 0.00025m, 0.0005m, 0.00075m, and 0.001m). switch on the computer and the instrument powers; wait for 30 minutes for 'warm up' of the instrument. 3.

Comments are closed.