Determination Of Concentration Of Kmno4 Soution Using Ferrous Ammonium Sulphate Meity Olabs

Determination Of Concentration Of Kmno4 Soution Using Ferrous ођ This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.co.in ?sub=73&brch=8&sim. This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.edu.in ?sub=73&brch=8&si.

Determination Of Concentration Of Kmno4 Soution Using Ferrous ођ It may also decompose into ferrous oxide when heated. as a result, this ferrous ammonium sulphate is not heated prior to titration. download class 12 chemistry viva questions on determination of concentration molarity of kmno4 solution by titrating it against a standard solution of – ferrous ammonium sulphate by clicking on the button below. Procedure: (a) preparation of 0.05m standard solution of ferrous ammonium sulfate: the quantity of mohr’s salt required for the 250ml of the solution having a normality of 0.05n can be calculated as follows. the molar mass of mohr’s salt = 392 g mol. strength = normality x equivalent weight. = (1 20) x 392 = 19.6 g l. Weigh 4.9 g of mohr’s salt on a weighing tube and dissolve it in 250ml of distilled water in a conical flask and add a little amount of concentrated h2so4 to prevent hydrolysis. when a solution with a concentration of 0.05m ferrous ammonium sulphate is titrated with a kmno4 solution, the following reaction occurs:. B. to determine the strength of the given kmno 4 solution using standard solution of mohr’s salt. materials required. real lab procedure. preparation of standard solution of mohr’s salt (ferrous ammonium sulphate).[250 ml m 20 (0.05 m) solution] using an electronic balance weigh exactly 4.9g of mohr’s salt crystals in a weighing bottle.

Determination Of Concentration Of Kmno4 Solution Meity Olabs You Weigh 4.9 g of mohr’s salt on a weighing tube and dissolve it in 250ml of distilled water in a conical flask and add a little amount of concentrated h2so4 to prevent hydrolysis. when a solution with a concentration of 0.05m ferrous ammonium sulphate is titrated with a kmno4 solution, the following reaction occurs:. B. to determine the strength of the given kmno 4 solution using standard solution of mohr’s salt. materials required. real lab procedure. preparation of standard solution of mohr’s salt (ferrous ammonium sulphate).[250 ml m 20 (0.05 m) solution] using an electronic balance weigh exactly 4.9g of mohr’s salt crystals in a weighing bottle. Ferrous ammonium sulphate (mohr’s salt) the theory what is titration? titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). the basis of this process is the reaction between the analyte and a solution of unknown concentration (standard solution). A. preparation of 0.05 m, standard solution of ferrous ammonium sulphate (molar mass of feso 4 (nh 4) 2 so 4 . 6h 2 o = 392 g mol –1). (i) weigh 4.9000 g of ferrous ammonium sulphate and transfer it into a 250 ml measuring flask through a funnel. (ii) transfer the solid sticking to the funnel with the help of distilled water into the flask.
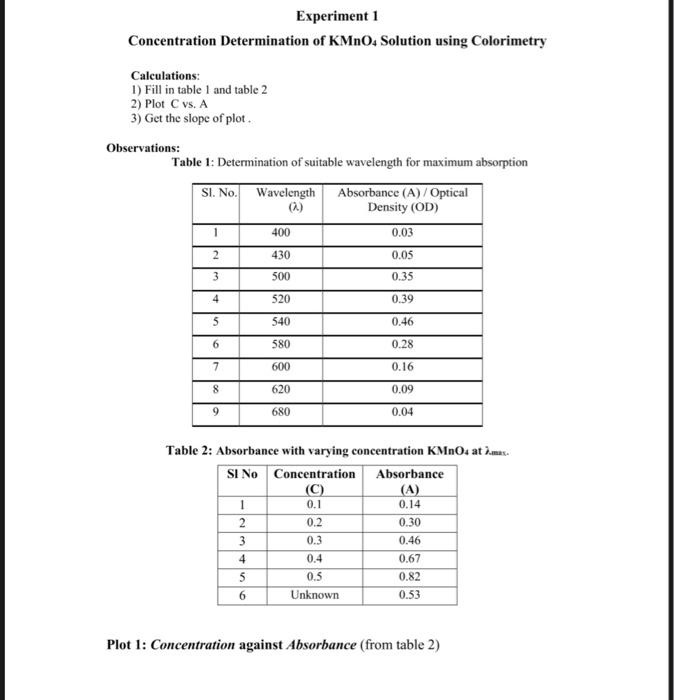
Solved Experiment 1 Concentration Determination Of Kmno4 Chegg Ferrous ammonium sulphate (mohr’s salt) the theory what is titration? titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). the basis of this process is the reaction between the analyte and a solution of unknown concentration (standard solution). A. preparation of 0.05 m, standard solution of ferrous ammonium sulphate (molar mass of feso 4 (nh 4) 2 so 4 . 6h 2 o = 392 g mol –1). (i) weigh 4.9000 g of ferrous ammonium sulphate and transfer it into a 250 ml measuring flask through a funnel. (ii) transfer the solid sticking to the funnel with the help of distilled water into the flask.

Comments are closed.