Determination Of Molarity Of Kmno4 Solution Using Standard Solution Of

Determination Of Molarity Of Kmno4 Solution Using Standard Solution Of Procedure: (a) preparation of 0.05m standard solution of ferrous ammonium sulfate: the quantity of mohr’s salt required for the 250ml of the solution having a normality of 0.05n can be calculated as follows. the molar mass of mohr’s salt = 392 g mol. strength = normality x equivalent weight. = (1 20) x 392 = 19.6 g l. Download class 12 chemistry viva questions on determination of concentration molarity of kmno4 solution by titrating it against a standard solution of – ferrous ammonium sulphate by clicking on the button below. download pdf. read also: mohr salt titration with kmno4; class 12 chemistry mcqs; class 12 chemistry important questions.

Determination Of Molarity Of Kmno4 Solution Using Standard Solution Of O What does it mean by standardization of potassium permanganate? (aim) it means to determine the strength of potassium permanganate with a standard solution of oxalic acid. this reaction helps to study the oxidation and reduction theory. tools and reagents for standardization of kmno4 : burette. distilled water. pipette. funnel. hot plate. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2 ) in an unknown solution. permanganate ion reduces to a manganese(ii) ion in the acidic solution. this reaction requires 5 electrons and 8 (!) hydrogen ions: mno 4 8h 5 e ® mn 2 4h 2 o. To prepare m 50 standard solution of mohr's salt and by using this solution find out the molarity and strength of the given kmno4 solution. ; practical : 24 prepare m 50 kmno4 solution and use this solution to find the molarity and strength of mohr's salt. A. preparation of 250ml of m 20 solution of mohr’s salt – the molar mass of mohr’s salt is 392gmol 1. it is a primary standard. since 1000cm 3 of 1m potassium permanganate require mohr’s salt of =392g so, 250cm 3 of m 20 potassium permanganate require mohr’s salt of = (392 20) 1000 × 250 = 4.9g.
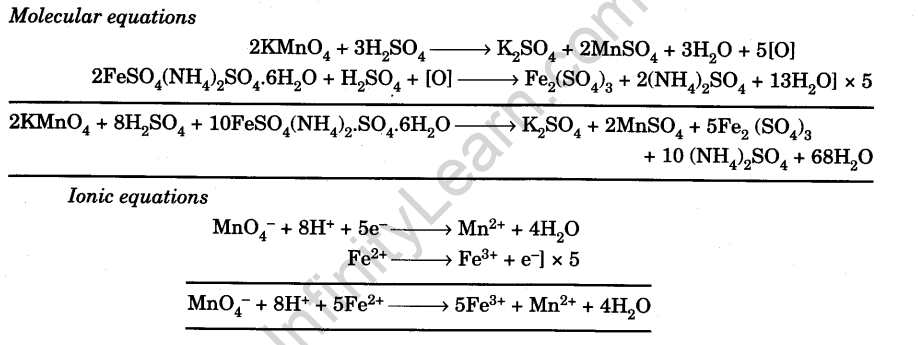
To Determine The Molarity Of Kmno4 Solution By Titrating It 44 Off To prepare m 50 standard solution of mohr's salt and by using this solution find out the molarity and strength of the given kmno4 solution. ; practical : 24 prepare m 50 kmno4 solution and use this solution to find the molarity and strength of mohr's salt. A. preparation of 250ml of m 20 solution of mohr’s salt – the molar mass of mohr’s salt is 392gmol 1. it is a primary standard. since 1000cm 3 of 1m potassium permanganate require mohr’s salt of =392g so, 250cm 3 of m 20 potassium permanganate require mohr’s salt of = (392 20) 1000 × 250 = 4.9g. The procedure for this experiment involves the preparation of a 0.05m standard solution of ferrous ammonium sulfate and the titration of the potassium permanganate solution against the standard ferrous ammonium sulfate (mohr’s salt) solution. it is essential to follow the steps carefully to achieve accurate results. Step 2: standardization of kmno4 solution: take 10 ml of n 10 n standard oxalic acid solution in a conical flask. add of 2 n h2so4 to the oxalate sample. heat the acidified oxalate solution to about 60 75 °c. record the initial burette reading. titrate the hot oxalate solution with the kmno4 solution until the appearance of a faint pink color.

Determime The Molarity Of Kmno4 Solution With The Help Of Supplied M The procedure for this experiment involves the preparation of a 0.05m standard solution of ferrous ammonium sulfate and the titration of the potassium permanganate solution against the standard ferrous ammonium sulfate (mohr’s salt) solution. it is essential to follow the steps carefully to achieve accurate results. Step 2: standardization of kmno4 solution: take 10 ml of n 10 n standard oxalic acid solution in a conical flask. add of 2 n h2so4 to the oxalate sample. heat the acidified oxalate solution to about 60 75 °c. record the initial burette reading. titrate the hot oxalate solution with the kmno4 solution until the appearance of a faint pink color.

Comments are closed.