Diagram Illustrating The Physical Changes Of State From Solid To Liquid
Properties Of Matter Mind Map A change of state is a physical change in a matter. they are reversible changes and do not involve any changes in the chemical makeup of the matter. common changes of the state include melting, freezing, sublimation, deposition, condensation, and vaporization. these changes are shown in the figure given below. By heating or cooling a substance, its state can be changed. there are four main. changes of state. : melting the process of a solid turning into a. liquid. close. liquid one of the three states.

States Of Matter A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid. 3 the triple point ( tp) of water is just 0.0075° above the freezing point; only at this temperature and pressure can all three phases of water coexist indefinitely. 4 above the critical point ( cp) temperature of 374°c, no separate liquid phase of water exists. figure 7.5.12 7.5. 12: phase diagram of co2 c o 2. A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. a phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. mesophases are of particular interest for liquid crystal technology. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist.
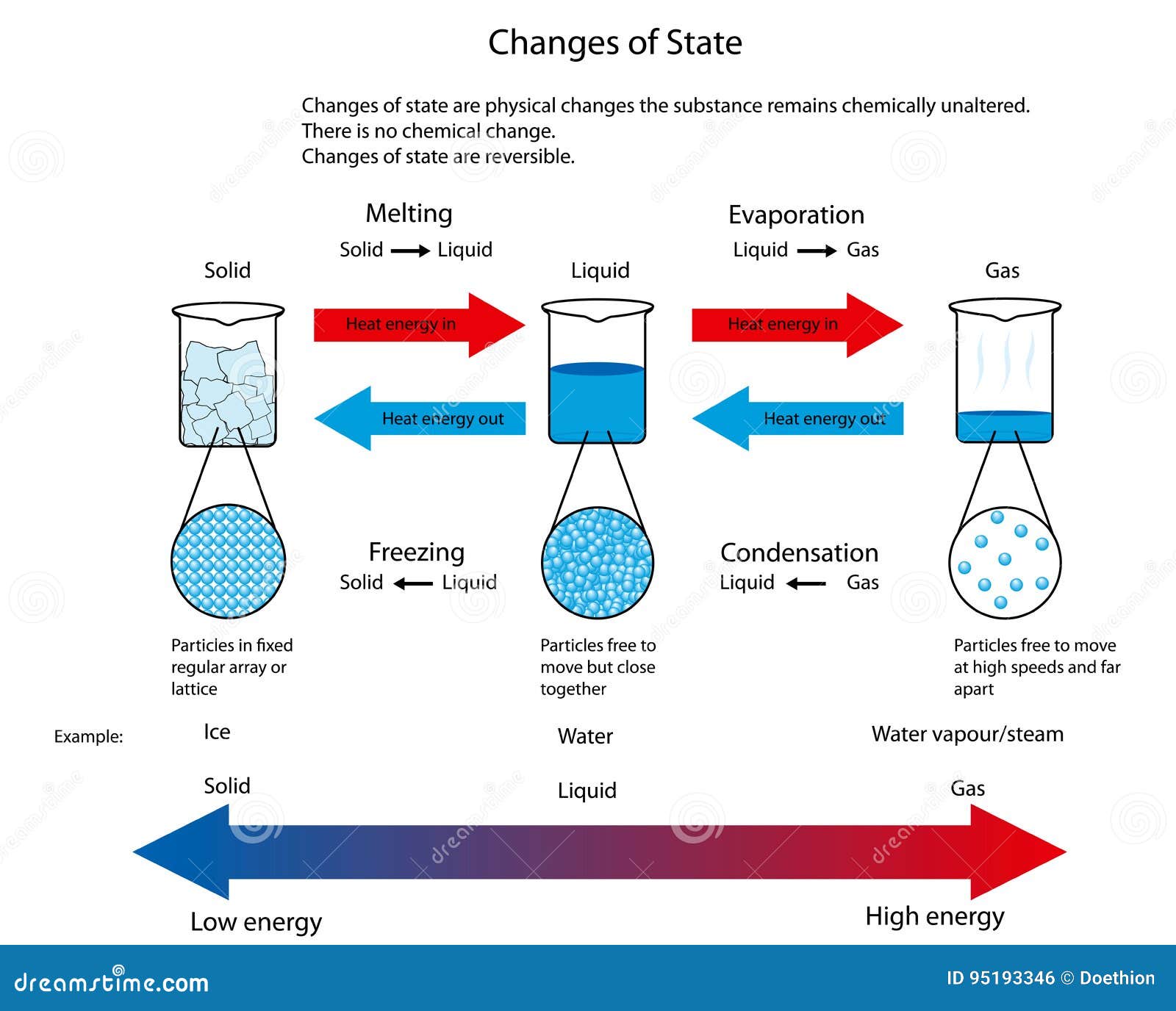
Particle Diagram For Solid Liquid And Gas A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. a phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. mesophases are of particular interest for liquid crystal technology. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic. the energy change associated with each common phase change is shown in figure 11.5.1. in chapter 9, we defined the enthalpy changes associated with various chemical and physical processes. The diagram summarises the common changes of state. the amount of energy needed to change state from solid to liquid, and from liquid to gas, depends on the strength of the forces between the.
2 1 2 Changes In The State Of Matter вђ Revision My Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic. the energy change associated with each common phase change is shown in figure 11.5.1. in chapter 9, we defined the enthalpy changes associated with various chemical and physical processes. The diagram summarises the common changes of state. the amount of energy needed to change state from solid to liquid, and from liquid to gas, depends on the strength of the forces between the.

Comments are closed.