Difference Between Physical Change And Chemical Change Comparison Between ођ

Chemical Change Definition For Kids Grade 5 Changes in matter: physical vs. chemical changes. Difference between physical and chemical change.
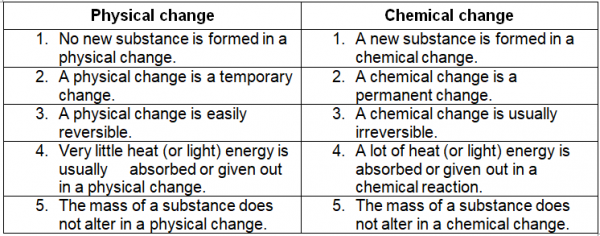
Difference Between Physical Change And Chemical Change Dunprime The difference between a physical change and a chemical change (or reaction) is composition. in a chemical change, there is a change in the composition of the substances in question; in a physical change there is a difference in the appearance, smell, or simple display of a sample of matter without a change in composition. Physical change refers to a change in which the molecules are rearranged but their internal composition remains same. chemical change is a process in which the substance transforms into a new substance, having different chemical composition. example. tearing of paper, melting freezing of water, cutting of trees, etc. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter. The difference between chemical and physical change explained. examples show how can you tell the difference between a chemical and physical change.
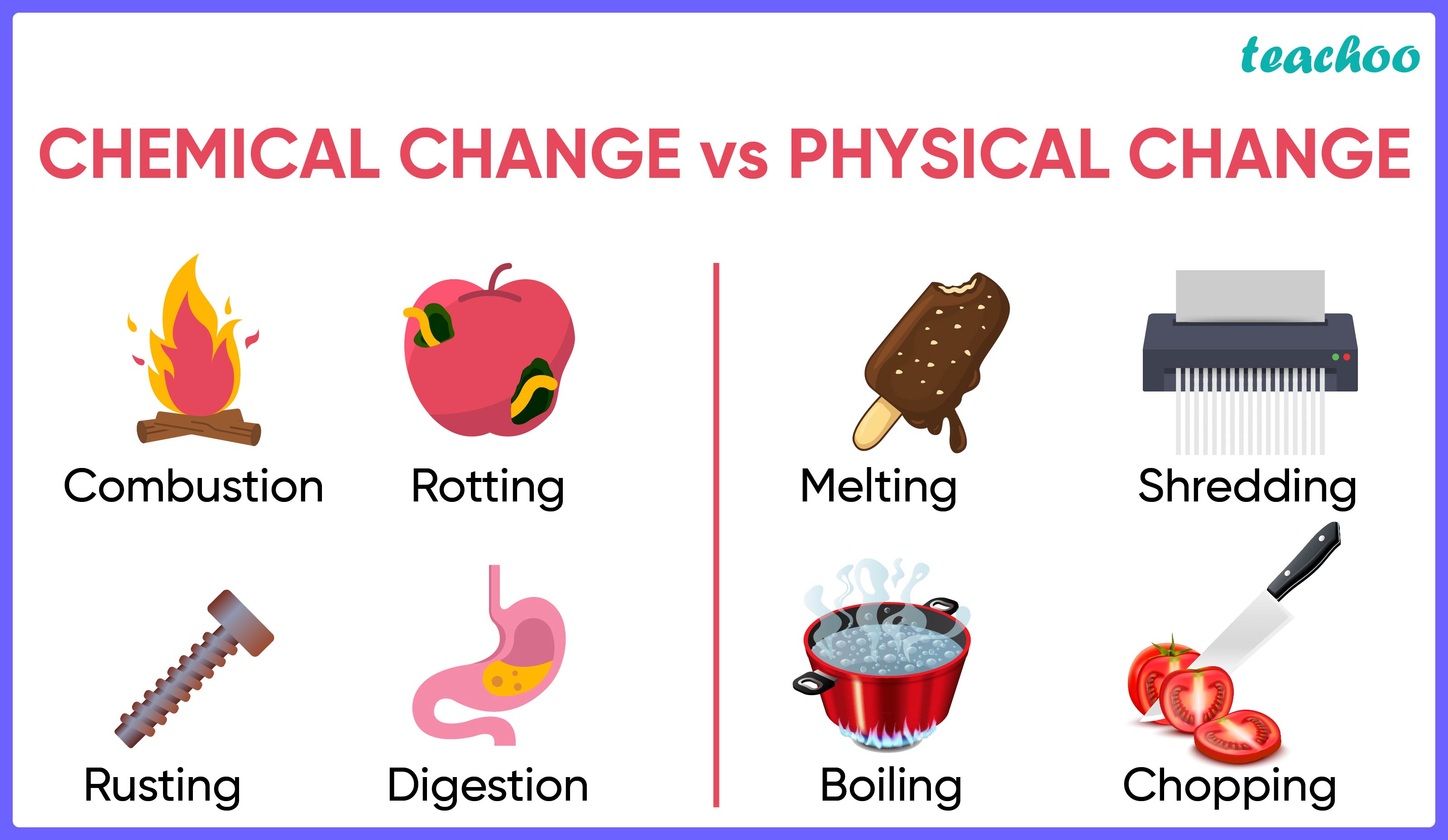
Difference Between Physical Change And Chemical Changes In Table Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter. The difference between chemical and physical change explained. examples show how can you tell the difference between a chemical and physical change. Describe the similarities and differences between physical and chemical changes; identify whether a physical or chemical change has taken place; suggest when a physical or chemical change may be useful. A chemical change can produce energy in the form of light, heat, sound, etc. examples of physical change: freezing of water into ice, ball milling, grinding, sublimation of camphor, boiling of water. examples of chemical change: burning of coal, formation of milk into curd, rusting of iron, digestion of food, etc.

Comments are closed.