Discovery Of Futibatinib

Discovery Of Futibatinib Youtube Deregulating fibroblast growth factor receptor (fgfr) signaling is a promising strategy for cancer therapy. herein, we report the discovery of compound 5 (tas 120, futibatinib), a potent and selective covalent inhibitor of fgfr1–4, starting from a unique dual inhibitor of mutant epidermal growth factor receptor and fgfr (compound 1). compound 5 inhibited all four families of fgfrs in the. Herein, we report the discovery of compound 5 (tas 120, futibatinib), a potent and selective covalent inhibitor of fgfr1 4, starting from a unique dual inhibitor of mutant epidermal growth factor receptor and fgfr (compound 1 ). compound 5 inhibited all four families of fgfrs in the single digit nanomolar range and showed high selectivity for.

Discovery Of Futibatinib The First Covalent Fgfr Kinase Inhibitor In Futibatinib (tas 120; taiho) is a novel, selective, pan fgfr inhibitor that inhibits fgfr1 4 at nanomolar concentrations. a framework for variation discovery and genotyping using next. Later phase futibatinib studies require serial liquid biopsies, and molecular characterization of these serial samples will provide insight into predictors of futibatinib sensitivity and resistance. in conclusion, futibatinib demonstrated clinical activity and a tolerable safety profile in heavily pretreated patients with advanced tumors in. Between april 16, 2018, and november 29, 2019, a total of 103 patients were enrolled and received futibatinib. a total of 43 of 103 patients (42%; 95% confidence interval, 32 to 52) had a response. Futibatinib, a potent, irreversible fgfr1 4 inhibitor. futibatinib is a structurally novel, highly selective, and potent fgfr inhibitor, 11 which binds covalently and irreversibly to a conserved cysteine residue in the fgfr kinase domain within the atp binding pocket 54 (fig. 2). as this cysteine residue is conserved across all fgfr receptors.

Aniruddha B S On Linkedin Drugdiscovery Covalentdrugs Medicine Between april 16, 2018, and november 29, 2019, a total of 103 patients were enrolled and received futibatinib. a total of 43 of 103 patients (42%; 95% confidence interval, 32 to 52) had a response. Futibatinib, a potent, irreversible fgfr1 4 inhibitor. futibatinib is a structurally novel, highly selective, and potent fgfr inhibitor, 11 which binds covalently and irreversibly to a conserved cysteine residue in the fgfr kinase domain within the atp binding pocket 54 (fig. 2). as this cysteine residue is conserved across all fgfr receptors. Herein, we report the discovery of compound 5 (tas 120, futibatinib), a potent and selective covalent inhibitor of fgfr1 4, starting from a unique dual inhibitor of mutant epidermal growth factor. Abstract. on september 30, 2022, the fda granted accelerated approval to futibatinib for the treatment of adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma (icca) with fgfr2 fusions or other rearrangements. approval was based on study tas 120–101, a multicenter open label, single arm trial. patients received futibatinib 20 mg.
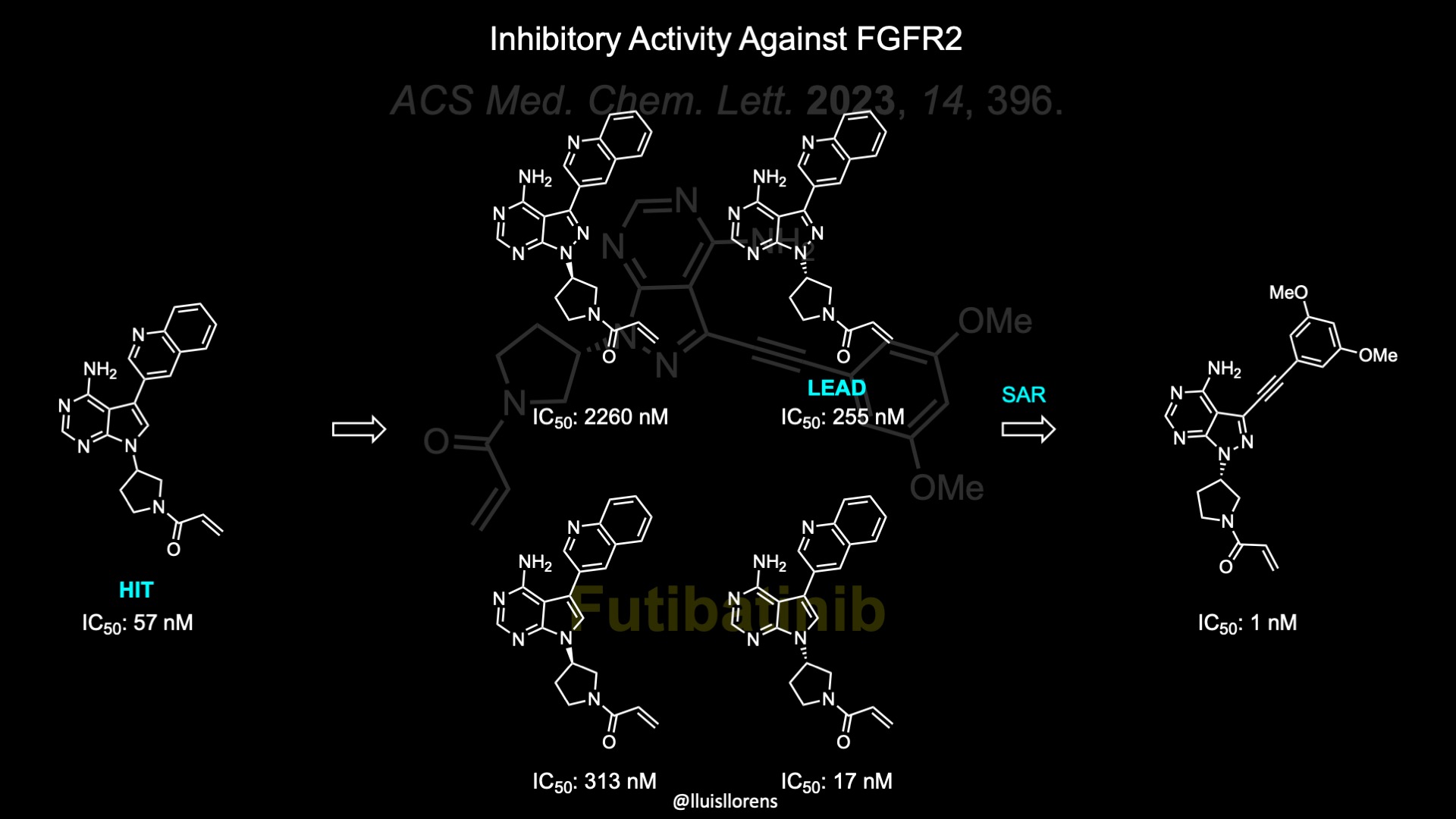
Futibatinib Mechanism Of Action And Synthesis Nrochemistry Herein, we report the discovery of compound 5 (tas 120, futibatinib), a potent and selective covalent inhibitor of fgfr1 4, starting from a unique dual inhibitor of mutant epidermal growth factor. Abstract. on september 30, 2022, the fda granted accelerated approval to futibatinib for the treatment of adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma (icca) with fgfr2 fusions or other rearrangements. approval was based on study tas 120–101, a multicenter open label, single arm trial. patients received futibatinib 20 mg.
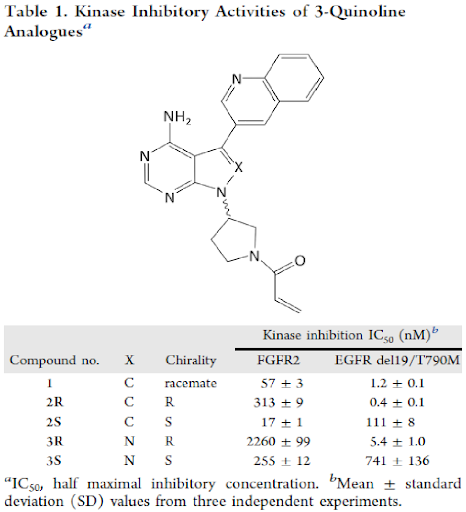
Discovery Of Futibatinib The First Covalent Fgfr Kinase Inhibitor In

Comments are closed.