Drug Development Clinical Trials Phases Of Clinical Trials Ind Nda New Drug Pharmacology
Navigating Clinical Trials Office For Clinical Research Advancement The data gathered during the animal studies and human clinical trials of an investigational new drug (ind) become part of the nda. the nda has evolved considerably during its history. Drug development plan and the role of the proposed study or clinical trial in that plan. the following list includes questions that should be considered during the pre ind ind period.

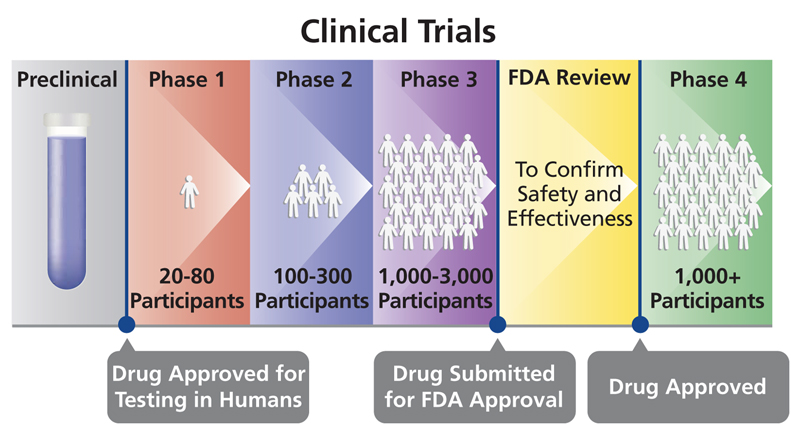
Drug Delivery Devices Throughout The Drug Development Cycle The four phases of a drug approval process includes: pre clinical, ind (investigational new drug) application. clinical. new drug application (nda) review. post marketing risk assessments. the full research, development and approval process can last from 12 to 15 years. The ind application must contain information in three broad areas: animal pharmacology and toxicology studies preclinical data to permit an assessment as to whether the product is reasonably. An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: ( 1) the consignee in the united states is the sponsor of the ind; ( 2) the consignee is a qualified investigator named in the ind; or. The ind stage consists of three phases. in phase i, clinical trials using healthy individuals are conducted to determine the drug’s basic properties and safety profile in humans. typically the drug remains in this stage for one to two years. in phase ii, efficacy trials begin as the drug is administered to volunteers of the target population.
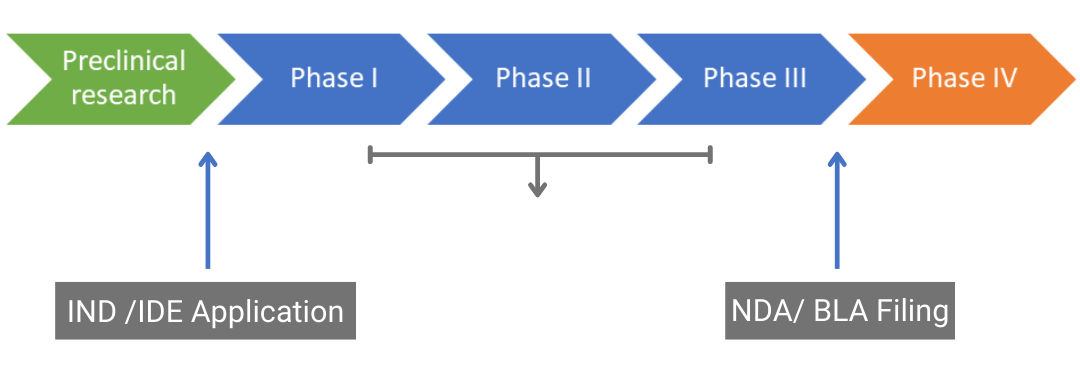
Planning Clinical Trial In Usa Here Is What You Should Know An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: ( 1) the consignee in the united states is the sponsor of the ind; ( 2) the consignee is a qualified investigator named in the ind; or. The ind stage consists of three phases. in phase i, clinical trials using healthy individuals are conducted to determine the drug’s basic properties and safety profile in humans. typically the drug remains in this stage for one to two years. in phase ii, efficacy trials begin as the drug is administered to volunteers of the target population. The process of discovering a new drug and conducting clinical trials to ensure it is safe and effective is estimated to take . 15 years . and cost . $2.6 billion. on average!!! • during the discovery process it takes 5,000 10,000 compounds to get just 5 that will be worthy advancing into the clinical development process (human studies) •. In the united states, the initial submission to permit use of an investigational drug in a clinical setting is called an investigational new drug (ind) application. in the european union, this documentation is submitted within a clinical trial application (cta). data required to support initial clinical trials.

Comments are closed.