Dur Asay Lal Asay
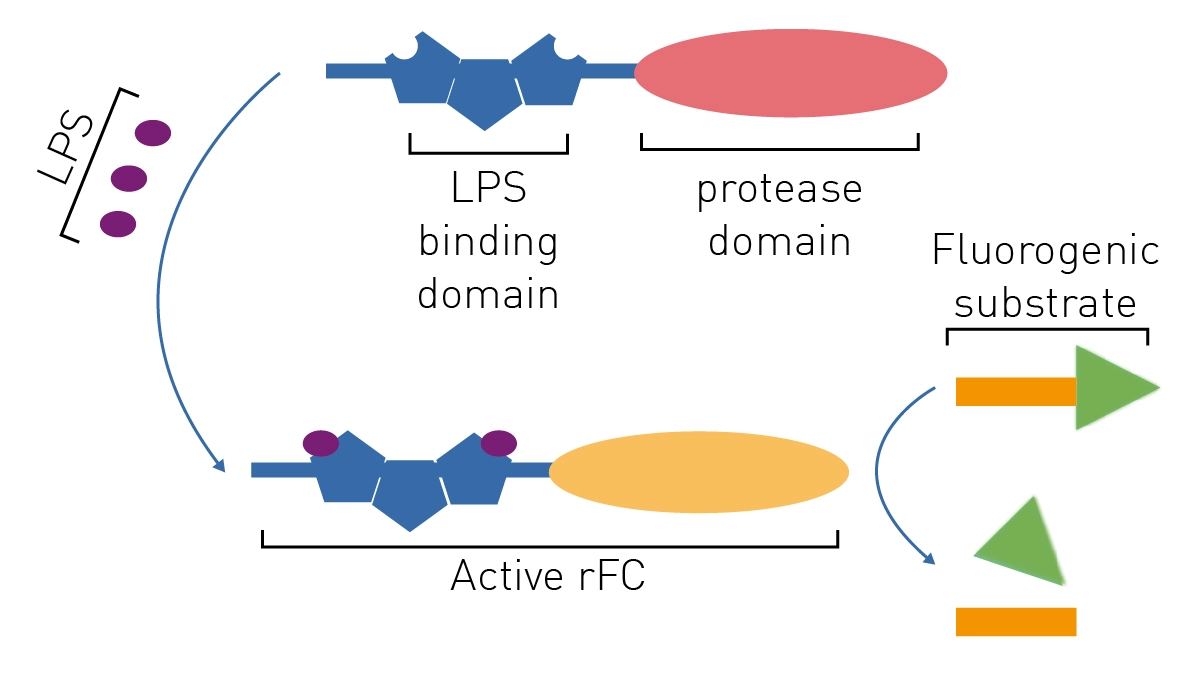
The Lal Assay Detecting Bacterial Contamination Bmg Labtech The most widespread endotoxin test is the lal test. the principle of the lal test is a reaction between lps and a substance (clottable protein) contained within amoebocyte cells derived from the blood of the horseshoe crab, as illustrated in figure 11.4 (of which limulus polyphemus is the most commonly used species, although other species, such as carcinoscorpius and tachypleus demonstrate the. Here the lal assay has a relatively high level of variability even for a biological assay. 1 this variation derives from 3 principle sources: reagents, product, and method. 2 this paper examines some of the reasons for lal test variation, focusing on photometric methods (chromogenic and turbidimetric), and considers how variation can be.

Professional 2022 High Quality Lal Assay Kit Mini Dry Heat Incubator Limulus amebocyte lysate (lal) is an aqueous extract of blood cells (amoebocytes) from the horseshoe crab, limulus polyphemus. lal reagent reacts with bacterial endotoxin and lipopolysaccharide (lps), which is a membrane constituent of gram negative bacteria. this reaction is the base on the lal reagent, which is then used for the finding and quantification of bacterial endotoxins. the gel. The lal assay has established a firm position as an alternative to the rabbit pyrogen test, and thus, the horseshoe crab has already proven to be an extremely beneficial organism for biomedical use. however, there is growing awareness of the importance of protecting endangered species, and thus, alternative assay technologies using recombinant. We next included glucan blocker and performed lal turbidimetric and chromogenic assays on samples 6 and 8. fig. 4 shows that blocking glucan reactivity in sample 6 causes a decrease in lal signal in all assays by a factor of 5 or more, while in sample 8, the reduction range is 25–47% depending on methods. download: download full size image. Limulus amebocyte lysate. limulus amebocyte lysate (lal) is an aqueous extract of motile blood cells (amebocytes) from the atlantic horseshoe crab limulus polyphemus. lal reacts with bacterial endotoxins such as lipopolysaccharides (lps), which are components of the bacterial capsule, the outermost membrane of cell envelope of gram negative.

Kinetic Lal Assay Quick Guide Lonza We next included glucan blocker and performed lal turbidimetric and chromogenic assays on samples 6 and 8. fig. 4 shows that blocking glucan reactivity in sample 6 causes a decrease in lal signal in all assays by a factor of 5 or more, while in sample 8, the reduction range is 25–47% depending on methods. download: download full size image. Limulus amebocyte lysate. limulus amebocyte lysate (lal) is an aqueous extract of motile blood cells (amebocytes) from the atlantic horseshoe crab limulus polyphemus. lal reacts with bacterial endotoxins such as lipopolysaccharides (lps), which are components of the bacterial capsule, the outermost membrane of cell envelope of gram negative. The lal test is a bacterial endotoxin test (bet) employed by medicinal product manufacturers worldwide. specifically, the lal test is a means of detecting and, in some cases quantifying the presence of endotoxins, also known as lipopolysaccharides (lps), deriving from gram negative bacteria. the lal test is employed as a batch release assay for. The cartridges contain 2 sample wells and 2 spiked wells, which allows the tests to be done automatically in duplicate, thus satisfying the harmonized usp eur. ph. bacterial endotoxin test (bet) for lal assessment. to perform a test, the user simply pipettes 25 μl of a sample into each of the four sample reservoirs of the cartridge.

Comments are closed.