Electron Configuration Of Copper Cu Lesson
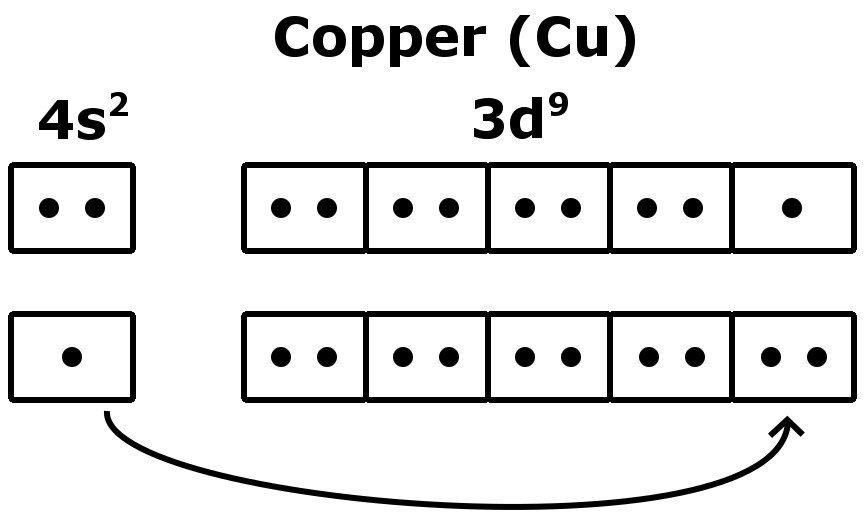
Copper Electron Configuration Cu With Orbital Diagram Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons. Atomic number, atomic weight and charge of copper ion. cu – 2e – → cu 2 . the electron configuration of copper ions (cu 2 ) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. copper atoms exhibit 1 and 2 oxidation states. the oxidation state of the element changes depending on the bond formation.

Electron Configuration Of Copper Cu Lesson Youtube The electron configuration of copper (cu) includes a fully filled 3d subshell. cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated with a half filled ( ns 1 , np 3 , nd 5 , nf 7 ) or filled ( ns 2 , np 6 , nd 10 , nf 14 ) subshell. What is the electron configuration of copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the only difference is at the end of the configuration that is in the 3d and 4s shells. The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. for nitrogen, the electron configuration is 1s2 2s2 2p3. this means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and three electrons in the 2p orbital. by understanding the rules of electron…. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. this unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. copper’s 3d¹⁰ configuration in the third energy level (shell.
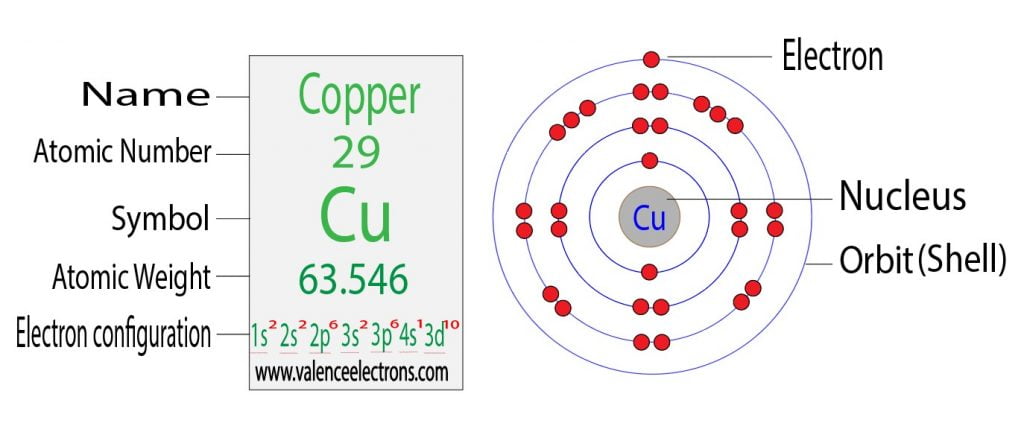
Electron Configuration For Copper Cu Cu Cu2 The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. for nitrogen, the electron configuration is 1s2 2s2 2p3. this means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and three electrons in the 2p orbital. by understanding the rules of electron…. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. this unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. copper’s 3d¹⁰ configuration in the third energy level (shell. The electron configuration and the orbital diagram are: following hydrogen is the noble gas helium, which has an atomic number of 2. the helium atom contains two protons and two electrons. the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = 1 2 m s = 1 2 ). The electronic configuration of copper (cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. this configuration indicates that copper has 29 electrons distributed in its electron shells. the first shell has 2 electrons, the second shell has 8 electrons, the third shell has 18 electrons, and the fourth shell has 1 electron.
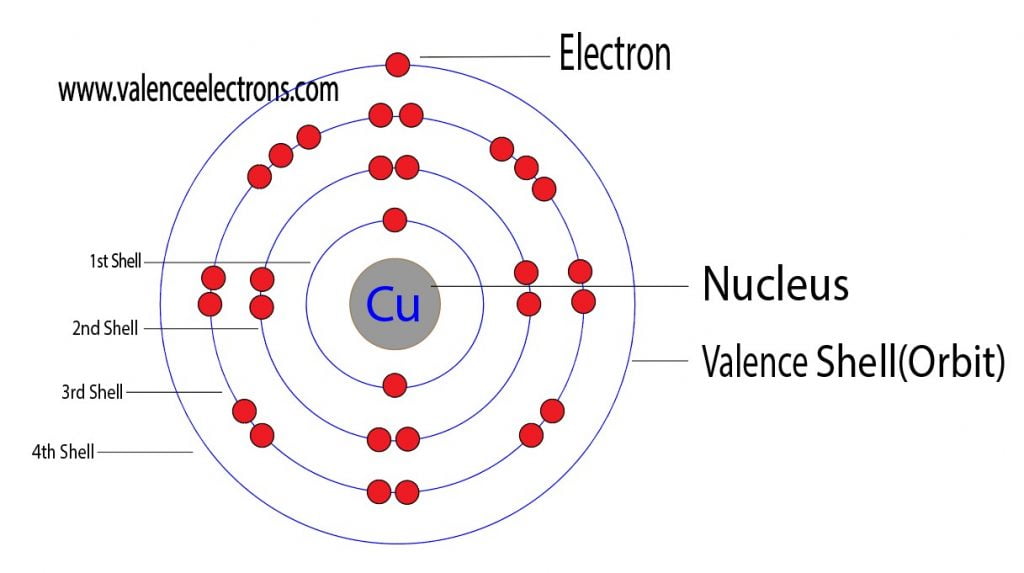
How Many Valence Electrons Does Copper Cu Have The electron configuration and the orbital diagram are: following hydrogen is the noble gas helium, which has an atomic number of 2. the helium atom contains two protons and two electrons. the first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = 1 2 m s = 1 2 ). The electronic configuration of copper (cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. this configuration indicates that copper has 29 electrons distributed in its electron shells. the first shell has 2 electrons, the second shell has 8 electrons, the third shell has 18 electrons, and the fourth shell has 1 electron.

Comments are closed.