Ethical Framework For Health Research

Having An Ethical Framework Is The Best Way To Mitigate Risks In An overview of ethics and clinical ethics is presented in this review. the 4 main ethical principles, that is beneficence, nonmaleficence, autonomy, and justice, are defined and explained. informed consent, truth telling, and confidentiality spring from the principle of autonomy, and each of them is discussed. The working group decided to broaden the scope of the 2002 guidelines from “biomedical research” to “health related research”. the working group considered biomedical research too narrow since that term would not cover research with health related data, for example. at the same time, the working.
The Principles Of Ethical Design And How To Use Them 99designs In addition, the framework’s guidance has not been specified for genomics research and public health research, which are essential for promoting global health equity. 48 in relation to the latter, principles and concepts from theories of health and social justice that focus on the structural determinants of health inequities can inform the development of guidance. 49–51 case studies of. Applying the ethical framework of the national commission to everyday clinical research, medical ethics scholars and irbs later analyzed questions such as: how can informed consent be a meaningful. Nih clinical center researchers published seven main principles to guide the conduct of ethical research: social and clinical value. scientific validity. fair subject selection. favorable risk benefit ratio. independent review. informed consent. respect for potential and enrolled subjects. The national research act of 19741 created the national commission for the protection of human subjects of biomedical and behavioral research.2 the act charged the commission with identifying the “basic ethical principles which should underlie the conduct of biomedical and behavioral research involving human subjects” and with developing associated guidelines for the ethical conduct of.
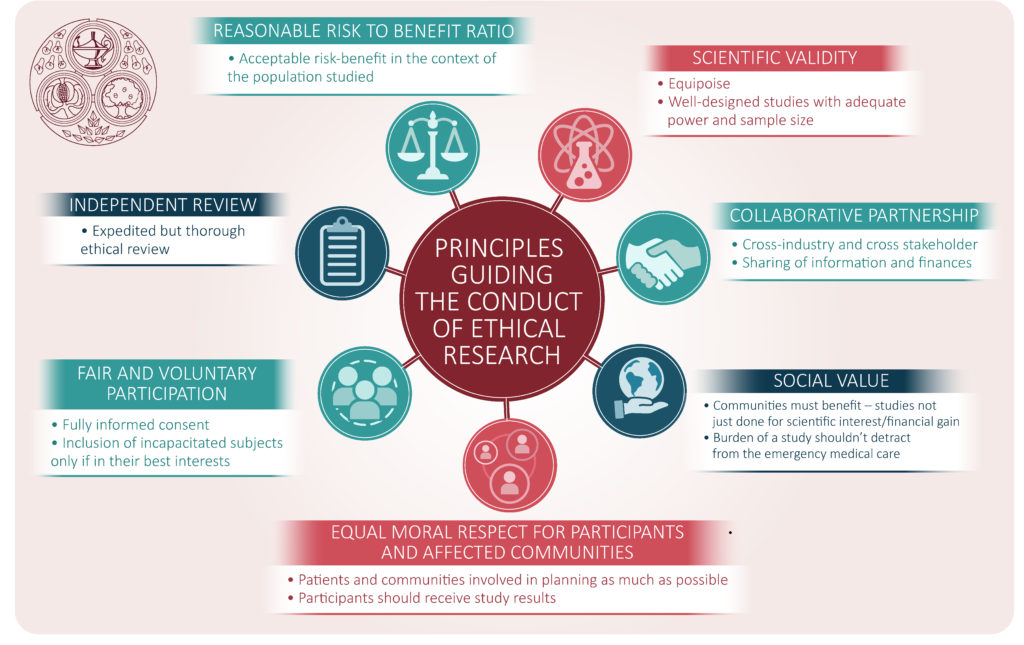
The Ethics Of Conducting Clinical Trials In The Search For Treatments Nih clinical center researchers published seven main principles to guide the conduct of ethical research: social and clinical value. scientific validity. fair subject selection. favorable risk benefit ratio. independent review. informed consent. respect for potential and enrolled subjects. The national research act of 19741 created the national commission for the protection of human subjects of biomedical and behavioral research.2 the act charged the commission with identifying the “basic ethical principles which should underlie the conduct of biomedical and behavioral research involving human subjects” and with developing associated guidelines for the ethical conduct of. The research for health justice framework. the research for health justice framework aims to help global health researchers and funders systematically link their practice to advancing global health equity. the framework assumes that the value of justice is central to and should guide global health research. justice is understood in terms of equity. 1 see canadian institutes of health research act, statutes of canada, 2000, chapter 6; ethics framework a. importance of research and research ethics.

Comments are closed.