Eu Regulatory Framework Pri
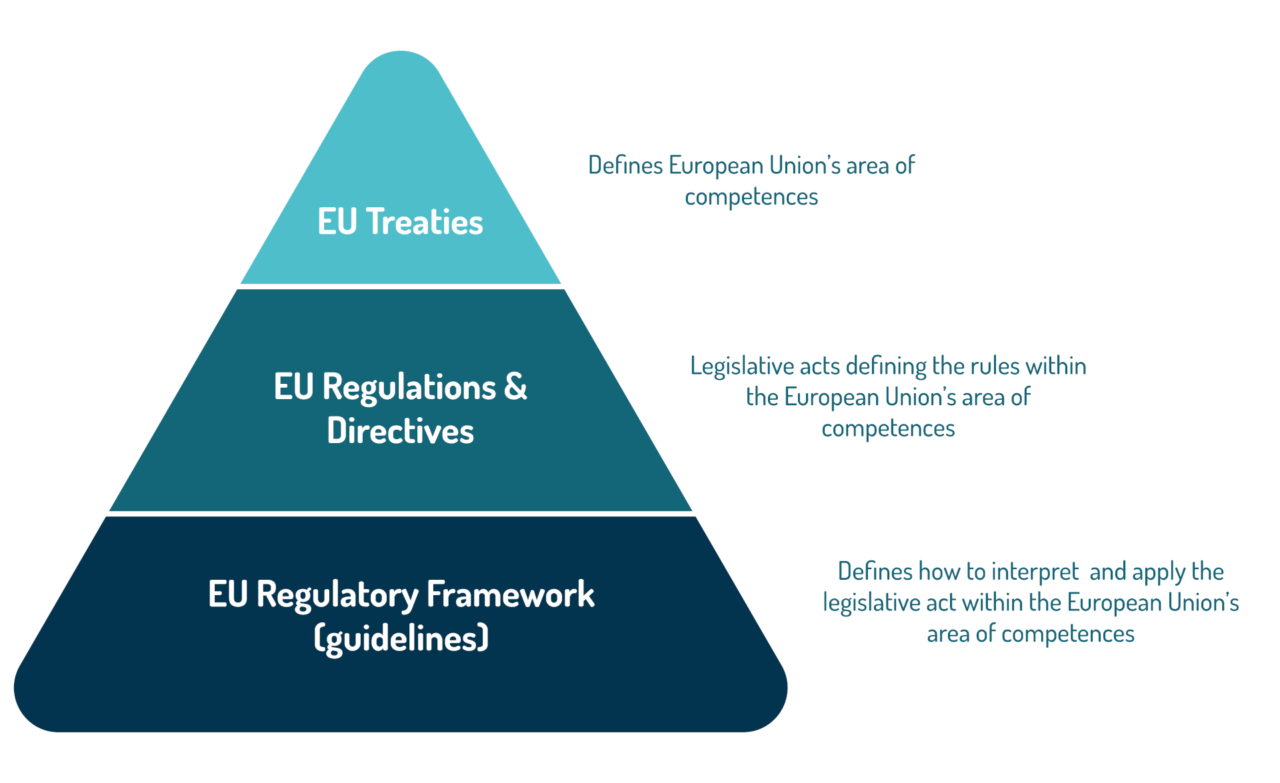
Eu Regulatory Framework Pri The treaty on the functioning of the european union (tfeu; also referred to as the treaty of rome) establishing the european economic community and creating a single market for goods, labour, services, and capital across member states. the aims set out in the eu treaties are achieved by several types of legal or legislative acts (eu legislation). The pri is the european microbiome regulatory science expertise center, created in 2010 to support the development and registration of therapeutic and diagnostic products emerging from microbiome science. we work to educate and contribute to the conditions of success for future medicinal innovations emerging from microbiome science, and our.
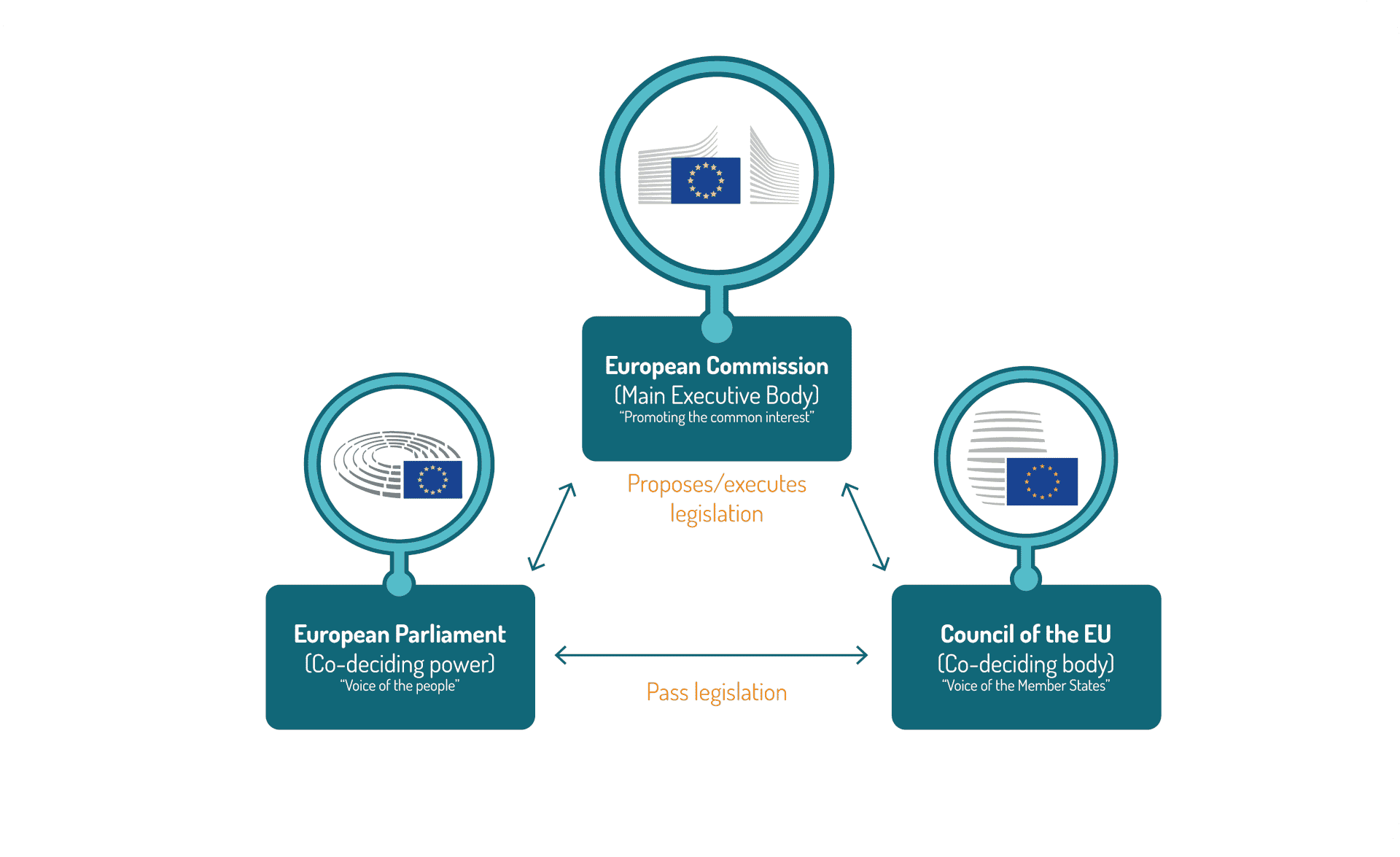
Eu Regulatory Landscape Pri This version incorporates the draft sfdr regulatory technical standards (rts) on the content, methodologies and presentation of disclosures, published on 2 february 2021, and draft rts on product disclosures related to the eu taxonomy, published on 22 october 2021. As the eu microbiome regulatory science center, the pri actively collaborates with experts from among its member organizations from industry and academia in order to help advance the state of the art for microbiome regulatory science standards and methods. these peer reviewed regulatory science publications represent a collective effort in. Pri’s regulation database documents financial, corporate and real economy policies which support, encourage or require responsible investment practice. this most recent update focuses on the top 20 countries by pri signatory count, plus the g20 members and the european union, to gather a comprehensive view of how policy frameworks are. On april 26, 2023, the european commission (the commission) published its long awaited proposed amendments to the eu regulatory framework for medicinal products (the proposals). this is the culmination of a number of years’ work by the commission, starting with the new pharmaceutical strategy for europe (the strategy) announced on november 25.

Comments are closed.