Fda Approves Medtronic Smart Cgm System For Diabetes
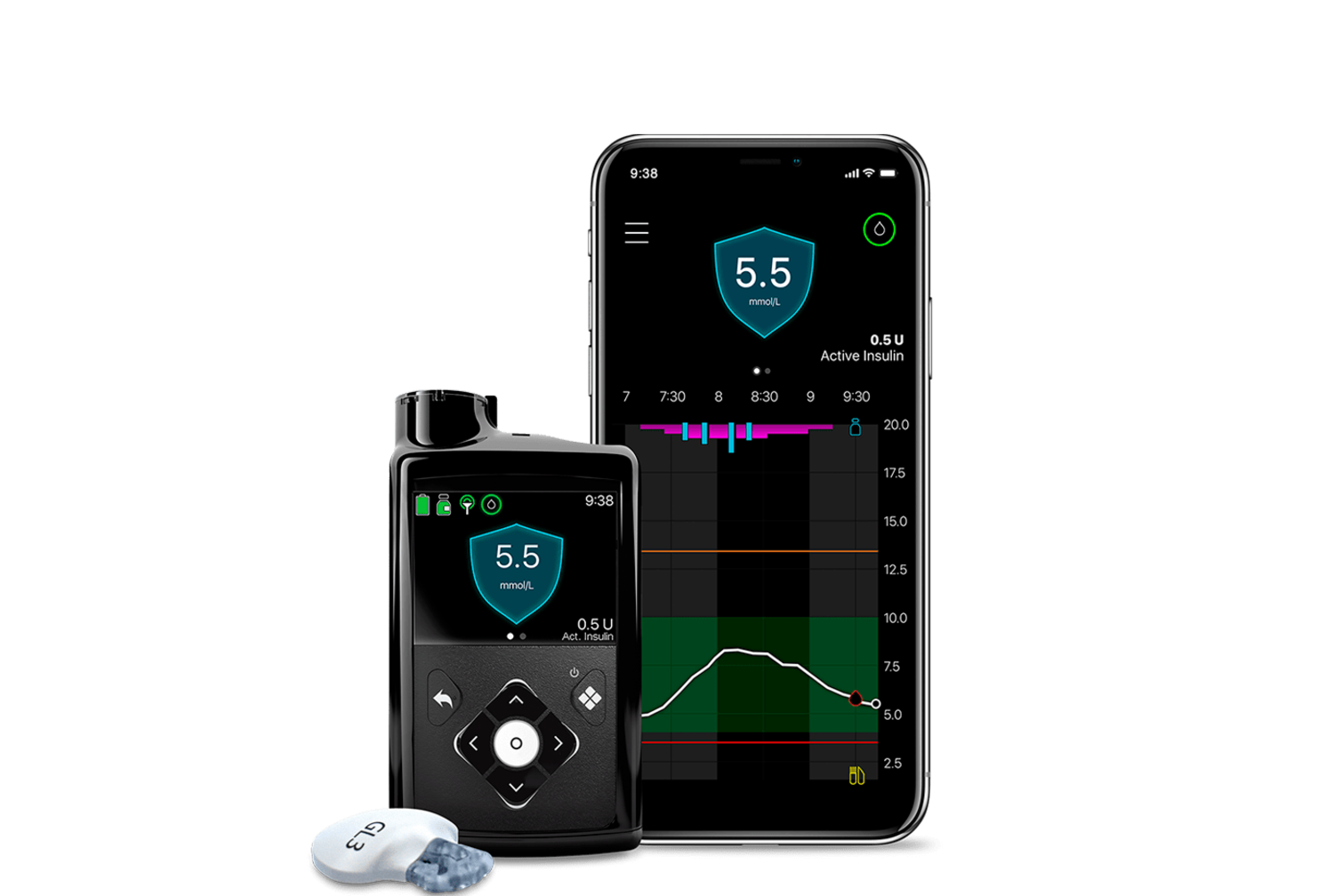
Continuous Glucose Monitoring Medtronic Diabetes The recent fda approval for simplera™ cgm lays the groundwork for future submission of the updated inpen™ smart insulin pen app, which would facilitate integration with simplera™ cgm, as a smart mdi system. once fda clearance is obtained, medtronic will initiate a limited market release in the u.s. beginning with existing standalone cgm. The guardian™ connect system continuously measures your glucose levels and delivers them right to your smartphone every 5 minutes. more importantly, it’s the only standalone cgm system that uses smart technology to predict where your glucose levels are headed. it alerts you from 10 60 minutes in advance that you’re predicted to hit a low.
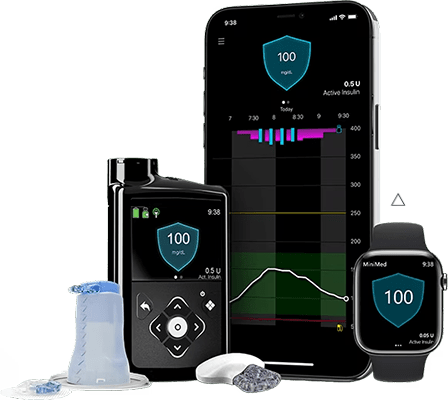
Continuous Glucose Monitoring Cgm Medtronic The simplera™ system requires a prescription and is indicated for the management of diabetes in persons ages 18 years and older. the sensor is indicated for up to 6 days of use plus an additional 24 hour grace period. blood glucose (bg) readings are needed (1) during the first 12 hours of use, (2) if not sensor data is available, (3) when. Medtronic diabetes announces world's first approval for. Guardian™ connect continuous glucose monitor. For diabetes. the us food and drug administration has approved a new stand alone continuous glucose monitoring (cgm) system, medtronic's guardian connect, that alerts users to impending high and.
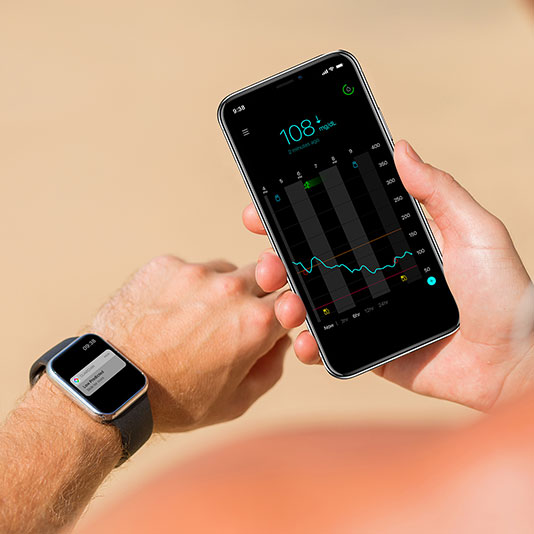
Guardian Connect юааcgmюаб юааsystemюаб Worldтащs First юааsmartюаб юааcgmюаб юааmedtronicюаб Guardian™ connect continuous glucose monitor. For diabetes. the us food and drug administration has approved a new stand alone continuous glucose monitoring (cgm) system, medtronic's guardian connect, that alerts users to impending high and. The fda has approved medtronic’s simplera continuous glucose monitor (cgm), with the company stating that it offers a smaller, more discreet, and user friendly device to help patients manage their diabetes. medtronic stated that the approval marks a significant advancement in cgm offerings by eliminating the need for overtape and simplifying. Guardian™ connect system continuous glucose monitor.
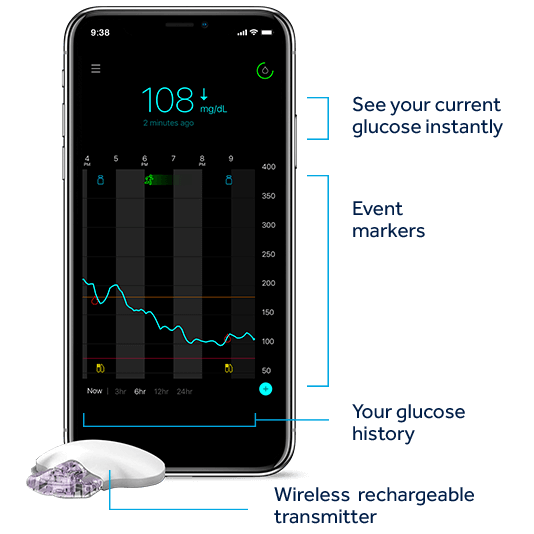
Guardian Connect юааcgmюаб юааsystemюаб Worldтащs First юааsmartюаб юааcgmюаб юааmedtronicюаб The fda has approved medtronic’s simplera continuous glucose monitor (cgm), with the company stating that it offers a smaller, more discreet, and user friendly device to help patients manage their diabetes. medtronic stated that the approval marks a significant advancement in cgm offerings by eliminating the need for overtape and simplifying. Guardian™ connect system continuous glucose monitor.

Comments are closed.