Fda Drug Approval Process Drugs

2021 Fda Drug Approval Process Infographic Conquer The Journey Informed The drug approval process takes place within a structured framework that includes: analysis of the target condition and available treatments —fda reviewers analyze the condition or illness for. Novel drugs at fda: cder’s new molecular entities and new therapeutic biological products. drug and biologic approval and ind activity reports. this week's drug approvals. drug trials snapshots.
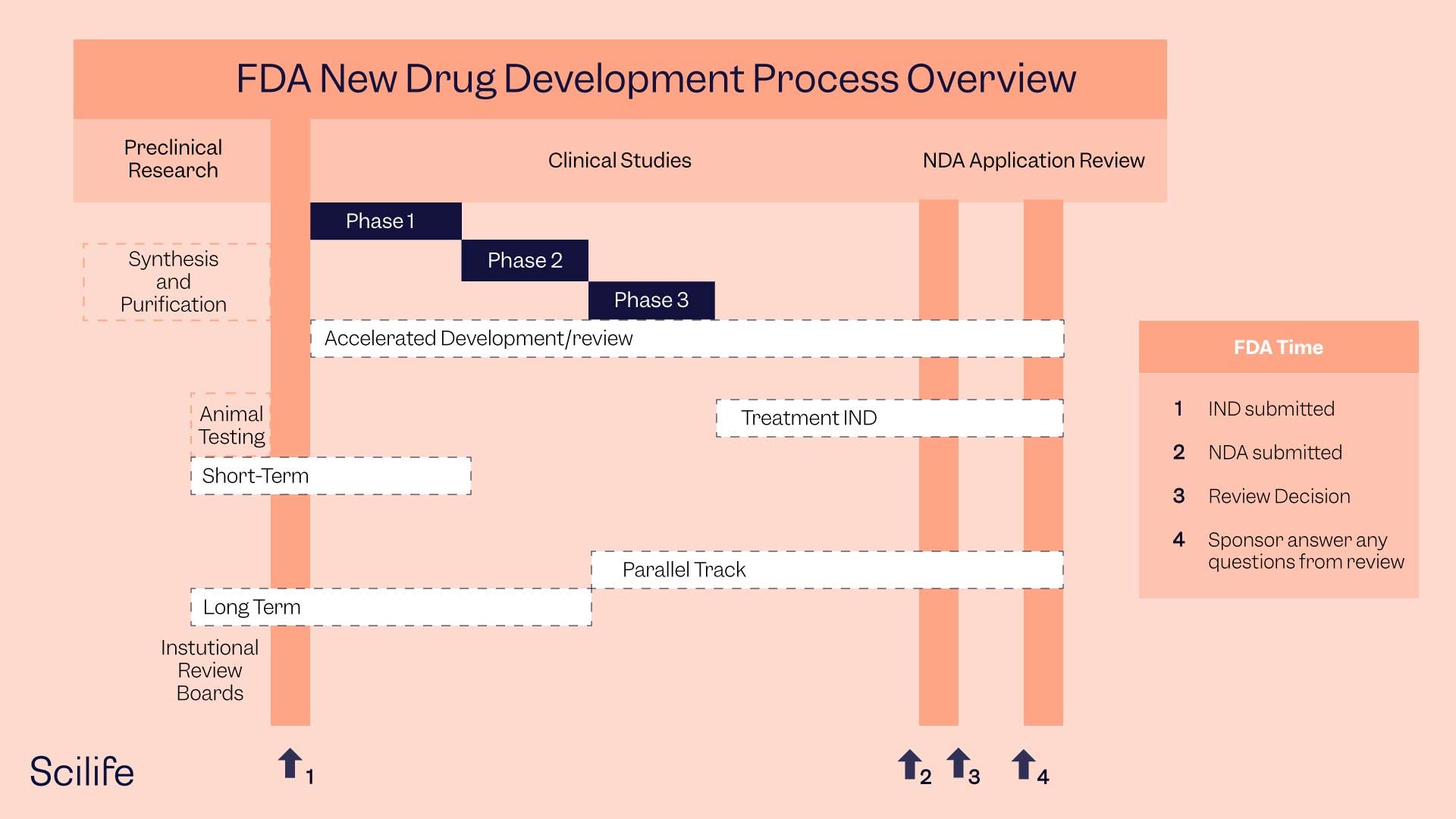
How To Get Fda Drug Approval Scilife The u.s. food and drug administration's (fda's) center for drug evaluation and research (cder) is a science led organization in charge of overseeing the drug approval process before a drug is marketed. cder ensures that both brand and generic drugs work correctly and that the health benefits outweigh the known risks. A: investigational new drug (ind) federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. new drug. A pharmaceutical company seeking fda approval to sell a new prescription drug must complete a five step process: discovery concept, preclinical research, clinical research, fda review and fda post market safety monitoring. first, the company must conduct laboratory tests and try the drug on animals and then people to make sure it works and is. Fda approved, fda acts through its postmarket or postapproval regulatory procedures. this report is a primer on drug approval and regulation: it describes (1) how drugs are approved and come to market, including fda’s role in that process and (2) fda and industry roles once drugs are on the pharmacy shelves. legislative history of drug regulation.

Pin On Infographics A pharmaceutical company seeking fda approval to sell a new prescription drug must complete a five step process: discovery concept, preclinical research, clinical research, fda review and fda post market safety monitoring. first, the company must conduct laboratory tests and try the drug on animals and then people to make sure it works and is. Fda approved, fda acts through its postmarket or postapproval regulatory procedures. this report is a primer on drug approval and regulation: it describes (1) how drugs are approved and come to market, including fda’s role in that process and (2) fda and industry roles once drugs are on the pharmacy shelves. legislative history of drug regulation. The fda approvals process is complex and time consuming, taking an average of 12 and 7 years for drugs and devices, respectively. source: getty images march 04, 2022 us food and drug administration approval processes comprise numerous steps to ensure the safety and efficacy of new drugs, therapies, and treatments. The drug development and approval process. the process of getting a drug to market, from first testing to final fda approval, is summarized in figure 1 and described at greater length below. drug companies continuously analyze thousands of compounds, seeking ones of therapeutic value. during the six to seven years of preclinical testing, the.

Comments are closed.