Find The Electron Configuration For Sulfur S And The Sulfide Ion

Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Sulfur is the sixth element in the third row of the periodic table.so it is the same as neon, but with a full 3s2 subshell and four electrons in its 3p.when. In this video we will write the electron configuration for s 2 , the sulfide ion. we’ll also look at why sulfur forms 2 ion and how the electron configurati.
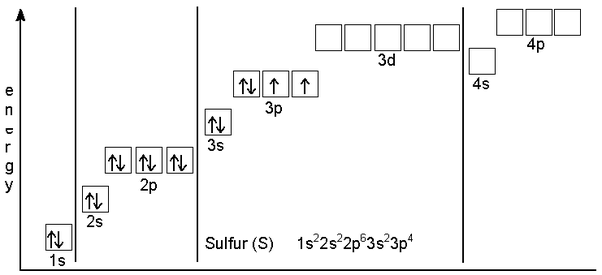
Sulfur Electron Configuration S With Orbital Diagram The electron configuration of a neutral sulfur atom will thus be "s: " 1s^2 2s^2 2p^6 3s^2 3p^4 now, the sulfide anion, "s"^ (2 ), is formed when two electrons are added to a neutral sulfur atom. as you can see in the configuration of the neutral atom, these two electrons will be added to the 3p orbitals, which can hold a maximum of six. The elements that receive electrons and form bonds are called anion. during the formation of sulfur bonds, the last shell of sulfur receives two electrons and turns into a sulfide ion(s 2 ). that is, sulfur is an anion element. s 2e – → s 2 the electron configuration of sulfide ion(s 2 ) is 1s 2 2s 2 2p 6 3s 2 3p 6. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). when we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the sulfur atom. in writing the electron configuration for sulfur the first two electrons will go in the 1s orbital. The electronic configuration of sulfur is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. electronic configuration of sulfide s 2 : sulfide ion is formed by the two electrons. the electronic configuration of sulfur ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. the 8 valence electrons in the sulfide ion encounter a large amount of repulsion between electrons than the 6.

S 2 Electron Configuration Sulfide Ion Electron Configuration In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). when we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the sulfur atom. in writing the electron configuration for sulfur the first two electrons will go in the 1s orbital. The electronic configuration of sulfur is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. electronic configuration of sulfide s 2 : sulfide ion is formed by the two electrons. the electronic configuration of sulfur ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. the 8 valence electrons in the sulfide ion encounter a large amount of repulsion between electrons than the 6. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the sulfide ion (s 2 ). from the periodic t. The br atom has 4s 2 3d 10 4p 5 as the electron configuration. therefore, br has 1 unpaired electron. answer (c): the b atom has 2s 2 2p 1 as the electron configuration. because it has one unpaired electron, it is paramagnetic. answer (d): the f ion has 2s 2 2p 6 has the electron configuration. because it has no unpaired electrons, it is.

Sulfur S Element 16 Of Periodic Table Elements Flashcards In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the sulfide ion (s 2 ). from the periodic t. The br atom has 4s 2 3d 10 4p 5 as the electron configuration. therefore, br has 1 unpaired electron. answer (c): the b atom has 2s 2 2p 1 as the electron configuration. because it has one unpaired electron, it is paramagnetic. answer (d): the f ion has 2s 2 2p 6 has the electron configuration. because it has no unpaired electrons, it is.

Comments are closed.