Gas Pressure Unit Conversions Torr To Atm Psi To Atm At
1 Atm Conversion Chart This video tutorial explains how to convert gas pressure units such as torr, atm, mm hg, kpa, and psi. examples include gas pressure conversions torr to atm. Next, let's look at an example showing the work and calculations that are involved in converting from torrs to atmospheres (torr to atm). torr to atmosphere conversion example task: convert 975 torrs to atmospheres (show work) formula: torr ÷ 760 = atm calculations: 975 torr ÷ 760 = 1.28289474 atm result: 975 torr is equal to 1.28289474 atm.
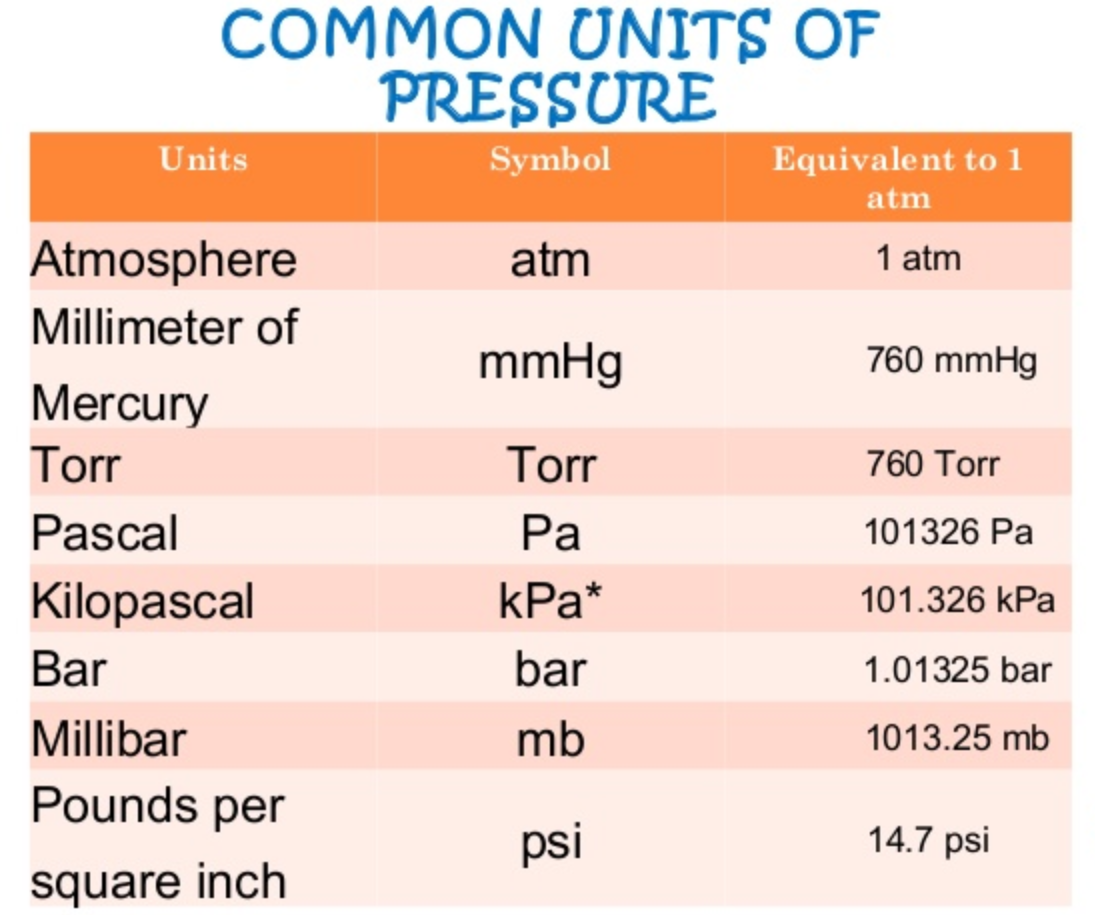
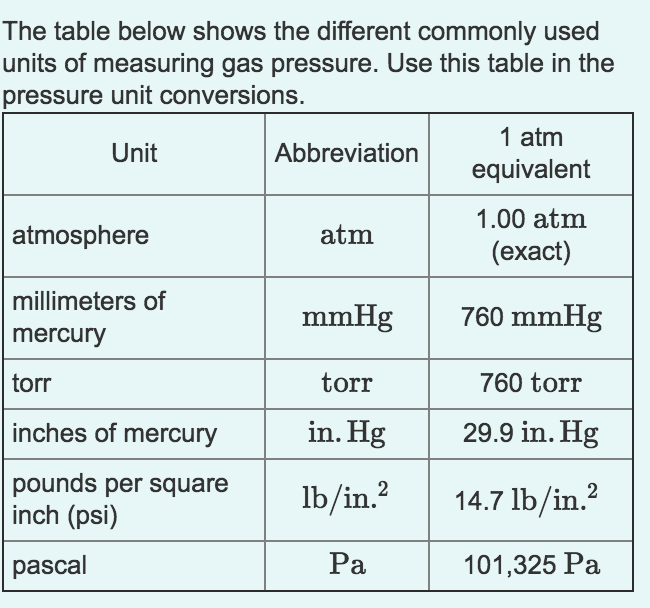
Unit To Measure Pressure According to the previous section's definition, there are exactly 760 760 760 torrs in one standard atmosphere (as one torr is precisely 1 760 of one atm). we can always convert this fraction to decimal, so the atm to torr conversion factor equals 0.00131578947 … 0.00131578947… 0.00131578947 …. The atmospheric pressure at sea level is 14.7 psi 14.7 psi. 1 atm = 760 mmhg = 760torr = 101.3kpa = 14.7psi 1 atm = 760 mm hg = 760 torr = 101.3 kpa = 14.7 psi. it is important to be able to convert between different units of pressure. to do so, we will use the equivalent standard pressures shown above. An atmosphere (or standard atmosphere) is a non si unit of pressure. the symbol for atmosphere is atm. there are 0.00131579 atmospheres in a torr. what is a torr (torr)? a torr is a non si unit of pressure. the symbol for torr is torr. there are 760 torrs in an atmosphere. Example #12: a student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 720.0 mmhg. express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. hint: 1 atm = 101.325 kpa = 760.0 torr = 760 mmhg = 14.696 psi = 101,325 pa solution: (720.0 mmhg) (1 atm 760 mmhg) = 0.9474 atm.

Comments are closed.