Gcse Chemistry Revision Transition Elements Triple

Gcse Chemistry Revision Transition Elements Triple Youtube Gcse workbooks amazon.co.uk dr shaun donnelly e b084fh9jpf?ref =dbs p pbk r00 abau 000000& encoding=utf8&tag=freesciencele 21&linkcode=ur2&linkid. Revise transition metals for your gcse chemistry foundation and higher triple science exams with bitesize interactive practice quizzes covering feedback and common errors.
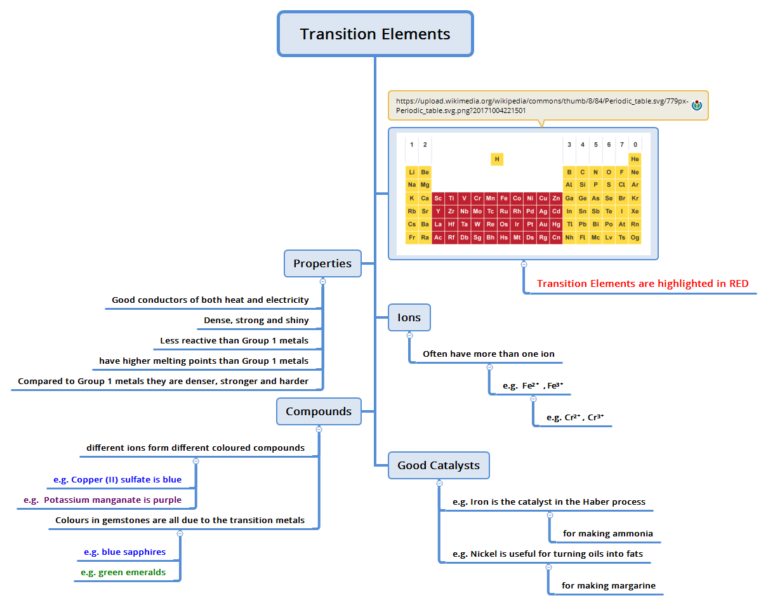
Gcse Chemistry Revision Transition Elements вђ E Chemistry Org U Chromium transition elements 1890°c 7.19 g cm 3 manganese transition elements 1240°c 7.20 g cm 3 iron transition elements 1538°c 7.87 g cm 3 cobalt transition elements 1492°c 8.90 g cm 3. Gcse; aqa; transition metals aqa physical properties of transition elements. the transition elements are metals. they have high melting points and densities, and are strong and hard. they form. Score maximum marks in your exams with our aqa gcse chemistry revision notes. written by teachers and examiners, they'll help you to fill knowledge gaps, understand complex concepts and boost your exam confidence. they’re designed for this specific exam board and syllabus, meaning you only revise what you need to know. General properties of transition metals. transition metals are elements that are found in groups 3 to 12 of the periodic table. they are characterised by their ability to form at least one stable ion with an incomplete d subshell. transition metals typically have high melting points and high densities due to the strength of the metallic bonds.

Transition Metals Alloys And Corrosion 4 Gcse Lessons Edexcel 9 1 Score maximum marks in your exams with our aqa gcse chemistry revision notes. written by teachers and examiners, they'll help you to fill knowledge gaps, understand complex concepts and boost your exam confidence. they’re designed for this specific exam board and syllabus, meaning you only revise what you need to know. General properties of transition metals. transition metals are elements that are found in groups 3 to 12 of the periodic table. they are characterised by their ability to form at least one stable ion with an incomplete d subshell. transition metals typically have high melting points and high densities due to the strength of the metallic bonds. The boiling points increase as you go down the group. how do the halogens react? they react to form ionic bonds with metals, with a 1 charge. what type of ions do group 1 elements form? 1 negative ion. study with quizlet and memorise flashcards containing terms like what is the charge of a proton?, true or false?. The transition metals are placed in the centre of the periodic table, between groups 2 and 3. they are generally hard and dense, and less reactive than the alkali metals. iron, copper, silver and gold are important transition metals. this video explains more about transition metals. gcse chemistry revision science section covering transition.

Comments are closed.