Gene Therapy For Sickle Cell Disease

Nih Researchers Create New Viral Vector For Improved Gene Therapy In Casgevy and lyfgenia are the first cell based gene therapies to treat patients 12 years and older with sickle cell disease and recurrent vaso occlusive crises. they use genome editing or gene delivery technologies to increase fetal hemoglobin or hemoglobin a t87q, which prevent red blood cell sickling. Fda approves first gene editing treatment for human illness.
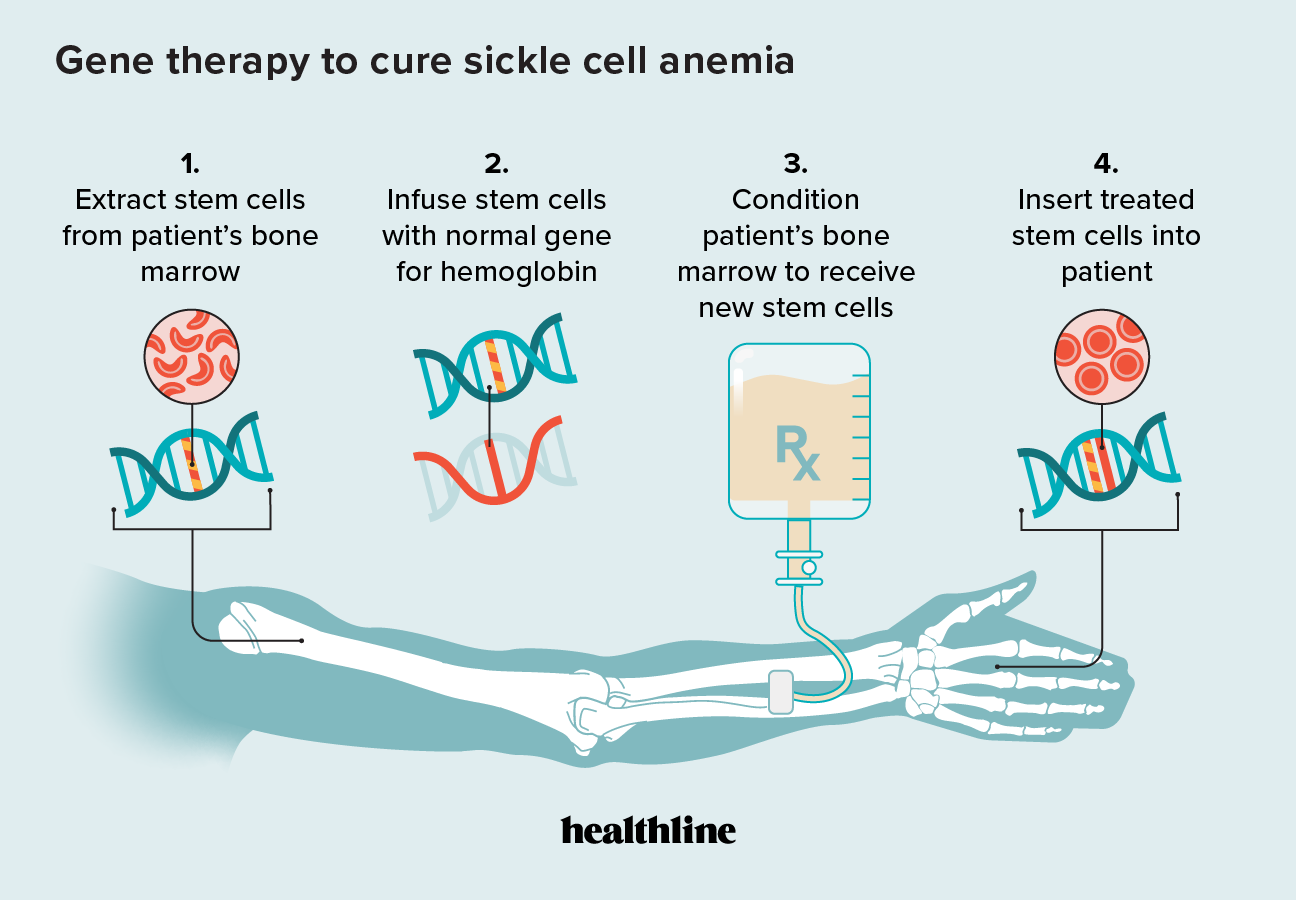
Sickle Cell Anemia And Gene Therapy How It Works Sickle cell disease (scd) has been well characterized for over 100 years, with the first clinical report published in 1910 describing it as the “first molecular disease.” 1 despite this long scientific history, progress toward identifying a cure has been slow, likely due in part to the fact that scd affects mostly individuals living in low resource settings or minority populations living. The u.s. national institutes of health (nih) celebrates the fda approval of two gene therapies for sickle cell disease, a hereditary illness affecting millions of people worldwide. nih has invested in basic and clinical research, education, and access to these treatments, and continues to support more research and innovation. First patient begins sickle cell gene therapy. Fda approves cure for sickle cell disease, the first.

Comments are closed.