Heat With Phase Change Worksheet

Heat With Phase Change Worksheet Now, add the amount of heat (q) from each part of the answer. total heat (q. t) = 12.54 kj 40.08 kj 6.15 kj = 58.77 kj. 6) how many joules are required to heat 75 grams of water from 85 ˚c to 185˚c? 251.845 kj start with specific heat because the water is frozen and must heat up from 85˚c to o˚c before it can go through a phase change. Table 11.3 latent heats of fusion and vaporization, along with melting and boiling points. let’s consider the example of adding heat to ice to examine its transitions through all three phases—solid to liquid to gas. a phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10.
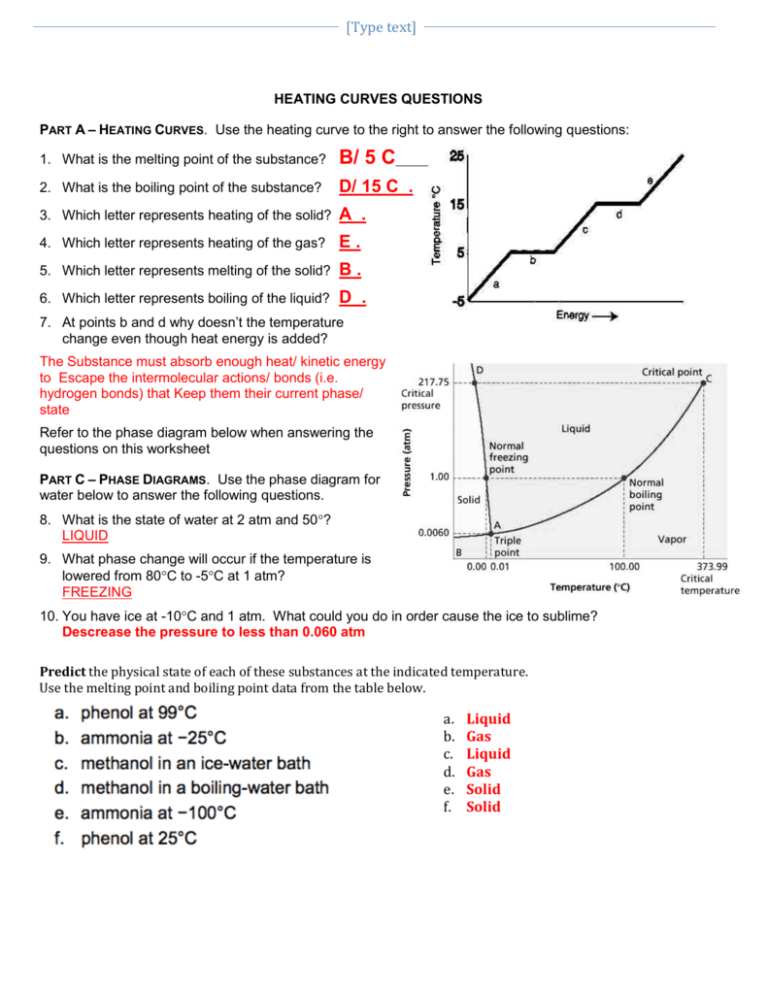
Heat With Phase Change Worksheet Explore how heat and temperature relate to phase changes. This phase change experiment? bonus: for water, the value for the latent heat of vaporization is 6.8 times greater than the latent heat of fusion. imagine we were adding heat at a constant rate to a block of ice in a beaker on a hot plate, and it took 4 minutes for the ice to melt completely. He temperature drops below about 4°c (a “hard freeze”). orchard owners sometimes spray a film of water ov. r the blossoms to protect the when a hard freeze is expected. from the poi. t. f view of phase changes, give a reason for the protect. on.3. heat transfer can cause tem. er. Phase change worksheet key. the graph was drawn from data collected as a substance was heated at a constant rate. use the graph and the words in the word bank to complete the statement. at point a, the beginning of observations, the substance exists in a solid state. material in this phase has definite volume and shape.
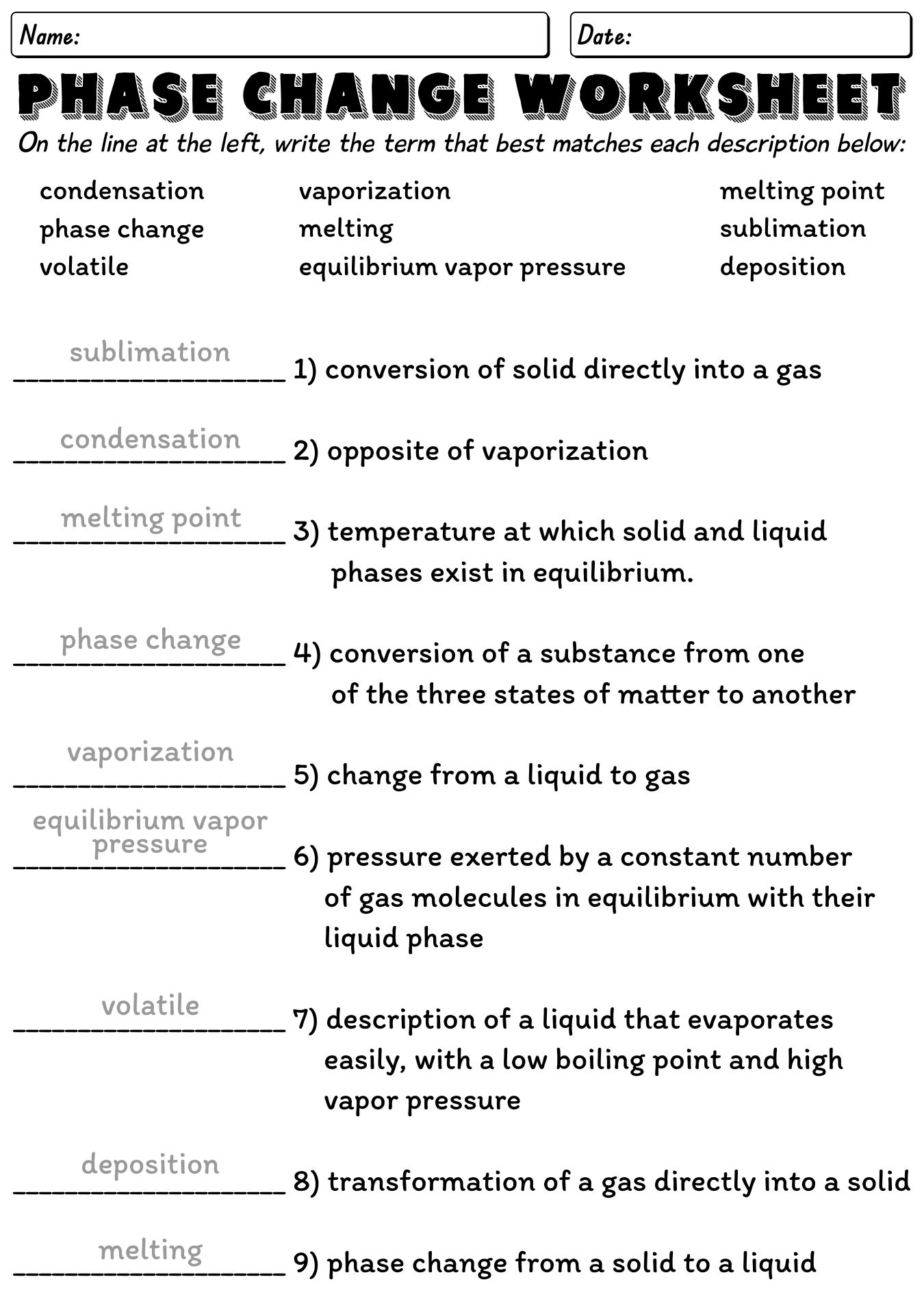
Phase Change Diagram Worksheet In this phase change experiment? bonus: for water, the value for the latent heat of vaporization is 6.8 times greater than the latent heat of fusion. imagine we were adding heat at a constant rate to a block of ice in a beaker on a hot plate, and it took 4 minutes for the ice to melt completely. how long would it take, after. This heat energy is called the latent heat of fusion. between 9 and 13 minutes, the added energy increases the temperature of the substance. during the time from point d to point e, the liquid is vaporizing. by point e, the substance is completely in the gas. phase. material in this phase has infinite volume and infinite shape.

Comments are closed.