Hemophilia A Gene Therapy Aav Mediated Delivery Of An Enhanced F8 Cassette To The
Jcm Free Full Text Emerging Immunogenicity And Genotoxicity An ideal disease for liver directed gene therapy. modest increases in factor viii levels (>1% of normal) have a positive impact on patient lives. adeno associated virus (aav) vectors have shown efficacy in preclinical and clinical studies; stable factor ix (fix) expression ~ five years in the clinic. lag in the clinic of aav human factor 8 (hf8. Adeno associated virus (aav)–mediated gene therapy is under investigation as a therapeutic option for persons with hemophilia a. efficacy and safety data include 3 years of follow up after a.
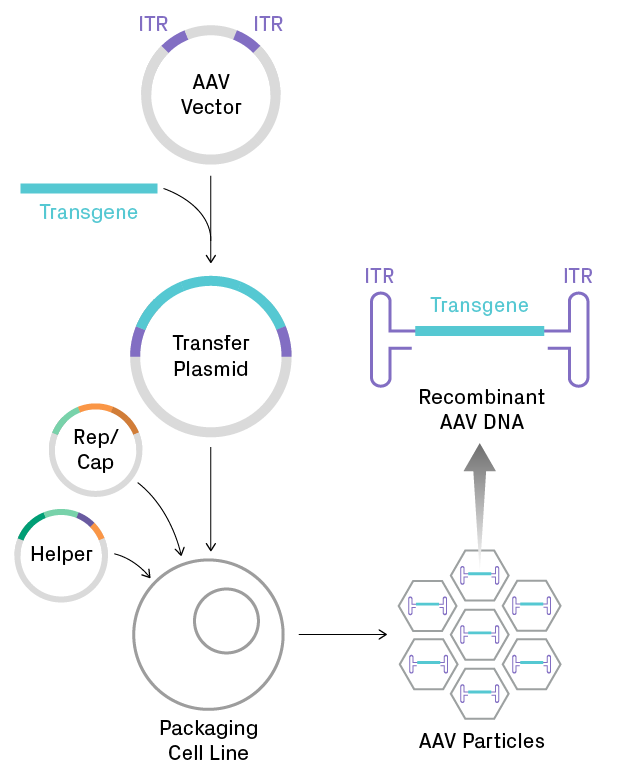
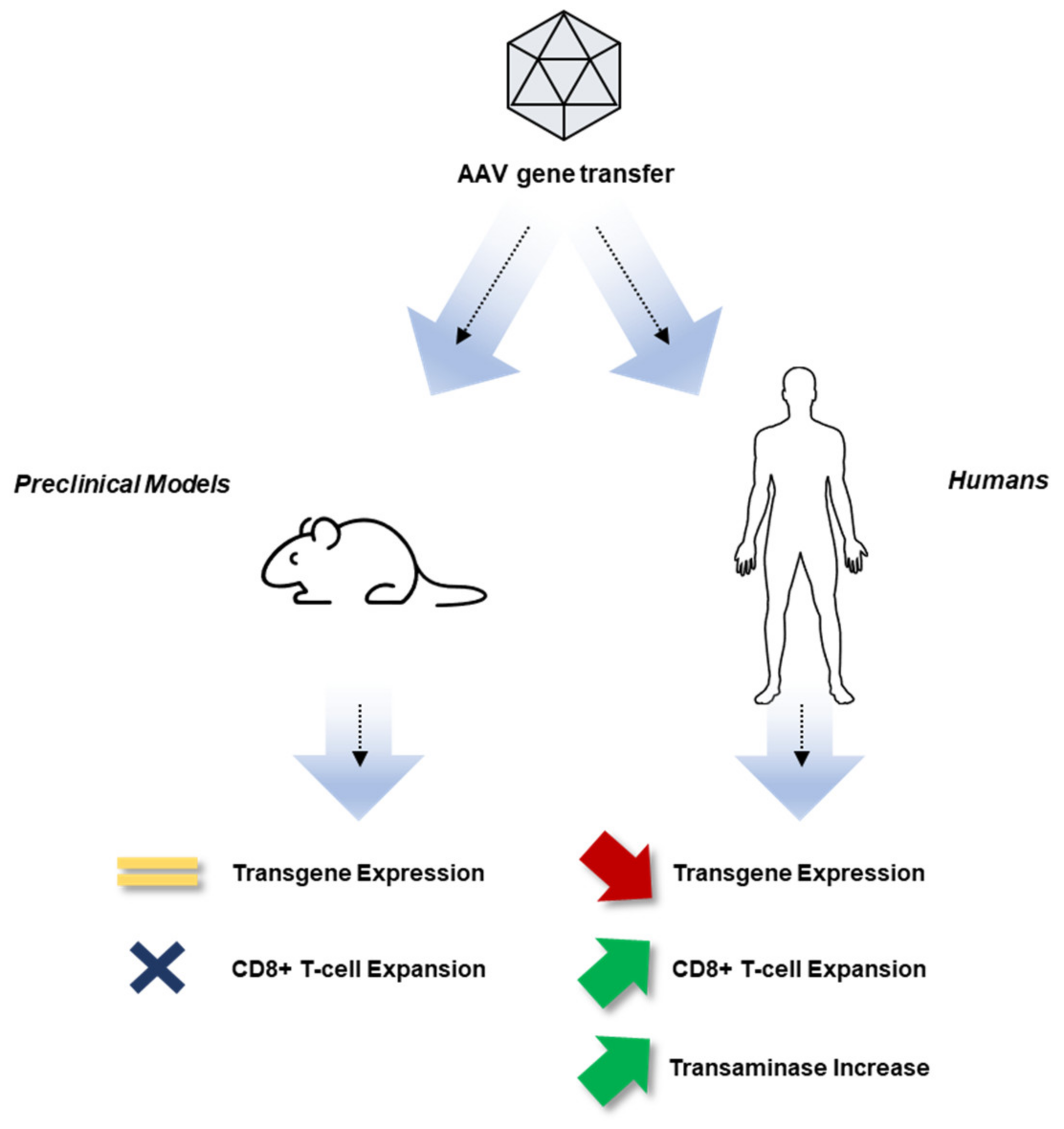
Aav Vectors In Gene Therapy Measuring Optimizing Quality Azenta In this phase 1–2 trial, we infused an investigational adeno associated viral (aav) vector (spk 8011) for hepatocyte expression of factor viii in 18 men with hemo philia a. four dose cohorts. Hemophilia a gene therapy: aav mediated delivery of an enhanced f8 cassette to the liver produces supraphysiological levels of human fviii in vivo additional information in the tuesday morning session on gene therapy, brigit e. riley explains her approach to the aav mediated delivery of an enhanced f8 cdna. One obstacle to efficacious aav gene transfer is a cellular immune response against the aav capsid, which was identified in trials of gene therapy for hemophilia b. 13,14 this response is vector. The general advantage of an enhanced function protein variant is exemplified by the success of second . generation hemophilia b (hb) gene therapies that universally employ a gain of function fix variant, fix padua17 19. in the absence of a naturally occurring enhanced function fviii variant, rationally engineering fviii.

Haemophilia Gene Therapy Institute Of Immunity And Transplantation One obstacle to efficacious aav gene transfer is a cellular immune response against the aav capsid, which was identified in trials of gene therapy for hemophilia b. 13,14 this response is vector. The general advantage of an enhanced function protein variant is exemplified by the success of second . generation hemophilia b (hb) gene therapies that universally employ a gain of function fix variant, fix padua17 19. in the absence of a naturally occurring enhanced function fviii variant, rationally engineering fviii. Aav vector liver directed gene therapy in hemophilia animal models induces immune tolerance to fviii and fix through induction of factor specific regulatory t cells (treg). 24 29 there is now strong evidence that aav and lentiviral (lv) vector liver directed gene therapy can also eliminate established inhibitors. 30 for instance, aav f8 and aav f9 vector liver gene transfer was an effective. Takeda’s gene therapy pipeline for hemophilia includes tak 754 (previously known as shp654 and bax 888), which is an aav serotype 8 vector that expresses fviii with the b domain deleted, for hemophilia a. they are conducting a phase 1 clinical study (study 201,501, nct03370172) that is active but not recruiting. 3.2.7.

Comments are closed.